��Ŀ����
��
�о���ѧϰ���⣺̽��ʵ�����о��õ�
NaOH�ı��ʳ̶����о��������ȳ�ȡ
13.3g��NaOH��Ʒ(����ΪNa2CO3)�������Һ��Ȼ������Һ��μ�����������Ϊ14.6g��ϡ���ᣬ��������CO2�������ⶨNa2CO3���������Ӷ���һ��ȷ����Ʒ��NaOH�ı��ʳ̶ȣ���������⡿ʵ���ü���ϡ��������������
CO2�����������ϵ����ͼ��ʾ����д�±���(����������С�������һλ)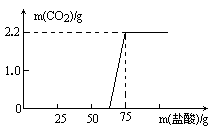

������̽��������ʵ���������
NaOH��Ӧ������������������������⡿���ݡ���
NaOH��Ӧ���������������������ͼ���㷢����ʲô���⣿
�𰸣�
������
������
|
����������⣺
��������̽���� m(NaOH)��13.3g��5.3g��8g���� NaOH��HCl��NaCl��H2O���� 40������36.5���� 8g������m(HCl)���� m(HCl)��8g������ m[HCl(aq)]�������������⣺ NaOH�������кͺ����μ����ᣬΪʲôû����������CO2���壿�������� |

��ϰ��ϵ�д�
 ȫ�̽��ϵ�д�
ȫ�̽��ϵ�д�
�����Ŀ
ѧУ�о���ѧϰС��ѡ��̽����CuSO4��Һ����ɫ��ʲô�����йء���Ϊ�о����⣬�����������ύ��ʵ�鷽�������в���Ҫ����ʵ���ǣ�������
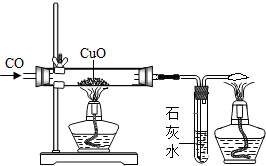 ij��ѧ�о���ѧϰС���ͬѧ��������ͼ��ʾ��̽��ʵ�飮ʵ�鷢��CO��CuO���Ⱥ��ɫ��ĩ��ɺ�ɫ��ĩ����������С����о����̲�����������
ij��ѧ�о���ѧϰС���ͬѧ��������ͼ��ʾ��̽��ʵ�飮ʵ�鷢��CO��CuO���Ⱥ��ɫ��ĩ��ɺ�ɫ��ĩ����������С����о����̲��������������о����⡿̽����ɫ��ĩ����Ҫ�ɷ�
���������ϡ�
��1���й����ʵ���ɫ��
Cu��ĩ����ɫ��CuO��ĩ����ɫ��
Cu2O��ĩ����ɫ��
��2��CuO��Cu2O���ܺ�ϡ���ᷢ����Ӧ����ѧ����ʽΪ��CuOʮH2SO4=CuSO4+H2O��Cu2O+H2SO4=CuSO4+Cu+H2O
��������ʵ�顿
��1�����Ӳ�ʲ������ں�ɫ��ĩΪһ�����ʣ���������ijɷ֣�����Ƽ�ʵ��֤����IJ²⣮
| ���� | ��ʵ�鷽�� | ���� | CO��CuO��Ӧ�Ļ�ѧ����ʽ |
A����ӦǰCuO��ĩ��������B��Ӳ�ʲ������й������ʼ��ٵ�����
C��ͨ��CO��������D����Ӧ������������������

 2Hg+O2��
2Hg+O2��