��Ŀ����
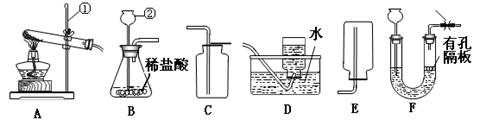
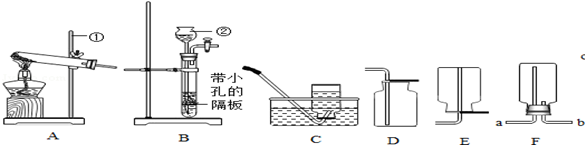
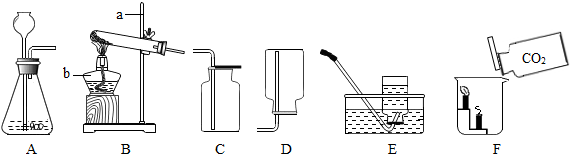
ʵ������ȡijЩ���������װ����ͼ��ʾ����ش��������⣺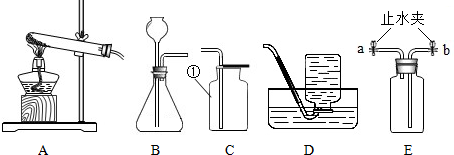
��1��ʵ�������ٵ�������
��2��ʵ������ȡ������̼����Ӧѡ��ķ���װ���� ����ѡ����ĸ������Ӧ�Ļ�ѧ����ʽΪ
��3��ѡ���ռ����巽��ʱ�����뿼�ǵ����������� ������ţ���
����ɫ ���ܶ� ���ܽ��� �ܿ�ȼ��
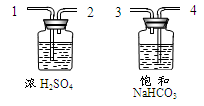
��4������װ��E��ˮ�ռ�������ƿ����װ��ˮ������� ���a����b��������ͨ��ƿ�ڣ�����װ��E��ȥ�����л���ˮ������ƿ��Ӧʢ�����������Լ� ����дѡ���ţ�
| A��Ũ���� | B��ϡ���� | C��ϡ���� | D��ʯ��ˮ |
��1������ƿ����2��B; CaCO3+2HCl�TCaCl2+H2O+CO2��;(3)�ڢ�;(4)a;A (5)D; ��
����

��ϰ��ϵ�д�
�����Ŀ