��Ŀ����
����ͼ��ʾװ�ý���CO��CO2�ķ�����������AΪ���ɼУ�BΪ��Һ©����������
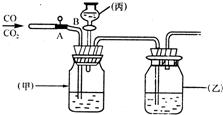
��֪��Ũ������к�ǿ����ˮ�ԣ�����������Һ���Կ������ն�����̼������������̼ͨ������������������Һ��������̼���ƺ�ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��1�����ƿ���ף���ʢ�� �����ƿ���ң���ʢ��Ũ���ᣬ�������� ����Һ©����������ʢ�� ��
��2���ȷ������������ ������Һ�彫��һ����������ʱ������Ӧ�Ļ�ѧ����ʽΪ ��

��֪��Ũ������к�ǿ����ˮ�ԣ�����������Һ���Կ������ն�����̼������������̼ͨ������������������Һ��������̼���ƺ�ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��1�����ƿ���ף���ʢ�� �����ƿ���ң���ʢ��Ũ���ᣬ�������� ����Һ©����������ʢ�� ��
��2���ȷ������������ ������Һ�彫��һ����������ʱ������Ӧ�Ļ�ѧ����ʽΪ ��
��ÿ��1�֣���6�֣�
CO2+2NaOH==Na2CO3+H2O
��1��NaOH��Һ �������� ϡ����
��2��CO Na2CO3+2HCl==2NaCl+H2O+CO2��
CO2+2NaOH==Na2CO3+H2O
��1��NaOH��Һ �������� ϡ����
��2��CO Na2CO3+2HCl==2NaCl+H2O+CO2��
����װ��NaOH��Һ����������CO2����ʱ�����г������DZ�����Ũ���������Ĵ�����CO����Ϊ������Ҫ�����CO��CO2���������ջ�Ҫ��Ѽ������յ�CO2�ٷų��������Ա���װϡ���ᣬ��������CO2��
�ʴ�Ϊ����1��NaOH��Һ����������,ϡ����
��2��CO��CO2+2NaOH==Na2CO3+H2O
�ʴ�Ϊ����1��NaOH��Һ����������,ϡ����
��2��CO��CO2+2NaOH==Na2CO3+H2O

��ϰ��ϵ�д�
�����Ŀ
 ��
�� �����ǵĵ�����������ͼ��ʾ�ı仯
�����ǵĵ�����������ͼ��ʾ�ı仯
