��Ŀ����
ijͬѧȥ���еĵ���ɽ�羰������ʱ��ȡ�������ɿ��ʯ��Ʒ�������������µķ�������Ʒ��̼��Ƶ������������м�⣺ȡ����ʯ��ʯ��Ʒ6�ˣ���40��ϡ������Ĵμ��룬���������������ݼ��±�����֪ʯ��ʯ��Ʒ�к��е����ʲ�����ˮ���������ᷴӦ������
��1��6��ʯ��ʯ��Ʒ�к��е�����Ϊ______�ˣ�
��2��m=______��
��3����Ʒ��̼��Ƶ�����������______��
��4��
| ����ϡ����Ĵ��� | 1 | 2 | 3 | 4 |
| ����ϡ������������ˣ� | lO | 10 | lO | lO |
| ʣ�������������ˣ� | 4.0 | m | O��6 | 0.6 |
�⣺��1���Ƚϵ����κ͵��Ĵε����ݿ�֪����Ʒ�����ʵ�����Ϊ0.6g��
��2���Ƚϵ�һ�κ͵����ε����ݿ�֪����һ�μ���10g�����ʣ����������Ϊ4.0g���������μ���ϡ�����ʣ����������Ϊ0.6g�����Կ����жϵ�һ����������ȫ��Ӧ������̼���6.0g-4.0=2.0g����10g ϡ�����ܹ�����2g̼��ƣ���˵ڶ�����Ҳ������2.0g̼��ƣ��ʿ������m=4.0-2.0=2.0��
��3��̼��Ƶ�����Ϊ��6.0g-0.6g=5.4g��������������Ϊ��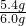 ��100%=90%
��100%=90%
��4�������������ΪX����
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73
2g X
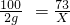
X=1.46 g��
������������������� ��100%=14.6%��
��100%=14.6%��
�ʴ�Ϊ����1��0.6��
��2��2.0��
��3��90%��
��4��14.6%��
��������1���Ƚϵ����κ͵��Ĵε����ݿ��Եó���Ʒ�����ʵ�����Ϊ��
��2���Ƚϵ�һ�κ͵����ε����ݿ�֪��һ����������ȫ��Ӧ������̼���6.0g-4.0=2.0g����˵ڶ�����Ҳ������2.0g̼��ƣ��ʿ������m��ֵ��
��3�������ʵ������������̼��Ƶ��������������̼��Ƶ�����������
��4�������������֪����һ�������2g̼���ǡ����ȫ��Ӧ�����Ծݴ�������������������
������������Ҫ����ѧ�����û�ѧ����ʽ������������ʽ���м���������������ʱѧ����Ҫ�������ͼ�����ݣ��������ʷ�Ӧʱ��������ϵ����ȷ���ù�ʽ�ͻ�ѧ����ʽ���н��
��2���Ƚϵ�һ�κ͵����ε����ݿ�֪����һ�μ���10g�����ʣ����������Ϊ4.0g���������μ���ϡ�����ʣ����������Ϊ0.6g�����Կ����жϵ�һ����������ȫ��Ӧ������̼���6.0g-4.0=2.0g����10g ϡ�����ܹ�����2g̼��ƣ���˵ڶ�����Ҳ������2.0g̼��ƣ��ʿ������m=4.0-2.0=2.0��
��3��̼��Ƶ�����Ϊ��6.0g-0.6g=5.4g��������������Ϊ��
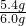 ��100%=90%
��100%=90%��4�������������ΪX����
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73
2g X
X=1.46 g��
�������������������
 ��100%=14.6%��
��100%=14.6%���ʴ�Ϊ����1��0.6��
��2��2.0��
��3��90%��
��4��14.6%��
��������1���Ƚϵ����κ͵��Ĵε����ݿ��Եó���Ʒ�����ʵ�����Ϊ��
��2���Ƚϵ�һ�κ͵����ε����ݿ�֪��һ����������ȫ��Ӧ������̼���6.0g-4.0=2.0g����˵ڶ�����Ҳ������2.0g̼��ƣ��ʿ������m��ֵ��
��3�������ʵ������������̼��Ƶ��������������̼��Ƶ�����������
��4�������������֪����һ�������2g̼���ǡ����ȫ��Ӧ�����Ծݴ�������������������
������������Ҫ����ѧ�����û�ѧ����ʽ������������ʽ���м���������������ʱѧ����Ҫ�������ͼ�����ݣ��������ʷ�Ӧʱ��������ϵ����ȷ���ù�ʽ�ͻ�ѧ����ʽ���н��

��ϰ��ϵ�д�
�����Ŀ
С��������ͬѧ�ԡ�Ѱ�ҳ������ܼ��ٹ�������ֽ�����ʣ���ͨ��ʵ��Ƚ����ǵ�Ч����Ϊ���չ�о���������ǻ�е�һЩ����ش�
��1���������о��ƻ�ʱ�����й۵㣬����Ϊ����ȷ����______
A��С����Ϊ����ͨ��ͼ��ݲ����������
B��С����Ϊ���Ե����Ŵ�ѧ���ר�ң��õ����ǵ�ָ��
C��С����Ϊ�������������������
D��С����Ϊ����Ҫ�������ϣ�ֻҪ����ʵ��Ϳ���ɱ��о�
��2�����������о�������ѡ�����������ʽ��������Ա�ʵ�飮����ʵ���鲻����Ϊ��Ӧ�IJ����ṩ֤�ݵ���______
| ��� | A | B | C | D |
| ���� | �ϻ��õĽ��������������Ч���� | �ϻ��õĽ������ʼ���Ч���� | �۵Ľ������������Ч���� | ������ȵ��ʼ���Ч���� |
| ʵ���� | ����������˿ | ͭ˿����˿ | ���������������� | ����ͭ��ͭ˿ |
A����ͬ��ʵ��ʱ����ʹ����Ͳ��ȡ��ͬ���Ĺ���������Һ
B����ͬ��ʵ��ʱ����ʹ��������ƽ��ȡ��ͬ���Ĺ�����Ʒ
C����ͬ��ʵ��ʱ������ͬ��ʵ���Ũ�ȡ�����������һ��
D����ͬ��ʵ��ʱ������һ��ʵ��ʹ�þƾ��Ƽ���
��4��������������Ƶ�����ʵ��װ�ã��������Ա�ʵ���У����й۲죨��ⶨ�����������ԱȽ���Ʒ�Թ�������ֽ����Ч������______
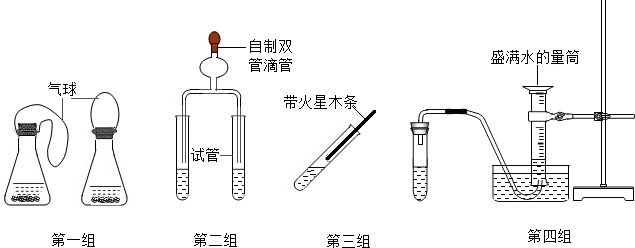
A����һ����ͬʱʵ�飬�۲�ȽϷ�Ӧ������������������ı仯���
B���ڶ�����ͬʱʵ�飬�۲�ȽϷ�Ӧ���������߲������ݵĿ���
C���������Ƿ�����ʵ�飬��ľ���Ƿ��ܸ�ȼ
D���������Ƿ�����ʵ�飬�ȽϷ�Ӧ�������ռ�һ��������������ʱ��
��5�����ǵ��о�������������˵��������Ϊ��ȷ����______
A������ͭ����������ͭ��ϡ���ᷴӦ�Ƶ�
B����������������������ȼ���Ƶ�
C��ͨ����ζԱ�ʵ�鶼��������ͭ�ļ���Ч������ͭ˿������ó��������ۣ�����ͭ�Թ��������ֽ�ļ������ú���ͭ˿
D�����������ļ���Ч��������˿���Ϳ��϶�������ļ������ú��ڵ��ʣ�