��Ŀ����
��8�֣���˹��ը�ѳ�Ϊ�����ҹ�ú���ش�����¹ʵġ�ͷ��ɱ�֡���
(1)��˹������ú�㼰��Χ�Ҳ��У��Ǿ����к�������ܳơ���˹����______ ______(ѡ����������)����˹����Ҫ�ɷ��Ǽ��飬����Ļ�ѧʽΪ ,��ȼ�յĻ�ѧ����ʽΪ ��
��2)��������˹��ըʱ�������Ծȴ�ʩ��������_______ __��
��3��ú����˹��ը������������������˹Ũ�ȴﵽ��ը�ȡ�_____ ______���㹻��___________�����κ�һ����������Ч���ƶ��ɱ�����˹��ը���ݴˣ��ҹ���ȫ��ܲ�������ˡ��ȳ��ɡ�����ء��Է綨���������ʩ��������еġ��ȳ��ɡ���ʩ���н��ͣ� __________��
(1)��˹������ú�㼰��Χ�Ҳ��У��Ǿ����к�������ܳơ���˹����______ ______(ѡ����������)����˹����Ҫ�ɷ��Ǽ��飬����Ļ�ѧʽΪ ,��ȼ�յĻ�ѧ����ʽΪ ��
��2)��������˹��ըʱ�������Ծȴ�ʩ��������_______ __��
| A��վ��ԭ�ز��� |
| B������ǰ��ˮ��Ӧ���Ի������ˮ�У�����ʪë����ס�ڱ� |
| C�����Ա�ը�ص�Ѹ���Ե� |
| D��ѡ�����ͨ��Ѹ������ |
��1������CH4��CH4+2O2 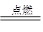 CO2 + 2H2O ��2��A
CO2 + 2H2O ��2��A
��3���¶ȴﵽ�Ż�㣬������������˹��Ũ�ȡ�
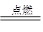 CO2 + 2H2O ��2��A
CO2 + 2H2O ��2��A��3���¶ȴﵽ�Ż�㣬������������˹��Ũ�ȡ�
�����������1����˹����Ҫ�ɷ��Ǽ��飬�Ǿ����к�������ܳƣ����ڻ���CH4�ڲ���ȫȼ�յ�����²���CO��H2O����Ӧ�ķ���ʽΪ CH4+2O2
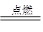 CO2 + 2H2O��
CO2 + 2H2O����2����������˹��ըʱ���ų��ж�����CO��Ҫ���Ծȿ��Ա��Ա�ը�ص�Ѹ���Ե�������ǰ��ˮ��Ӧ���Ի������ˮ�У�����ʪë����ס�ڱǣ�ѡ�����ͨ��Ѹ���������Ծȴ�ʩ��ȷ����B��C��D����ѡA��
��3������ȼ�պͷ�����ը�������������Ŀռ��ڣ���ȼ�����۳��������ϣ��ﵽ��ը���ޣ�������������ú����˹��ը��������������˹Ũ�ȴﵽ��ը�ȣ����������㹻���������ҹ���ȫ��ܲ���������ȳ��ɵĴ�ʩ��ҪĿ���ǣ�������˹��Ũ�ȣ��ʴ�Ϊ�����������������ȳ��ɣ�������˹��Ũ�ȣ�
������ͨ����˹��ը���¼�����������ȼ�ױ��İ�ȫ֪ʶ��Ӧ�ü�������ʩ��

��ϰ��ϵ�д�
 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д� ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д�
�����Ŀ
 + 5H2O
+ 5H2O

