��Ŀ����
�о������������ܹ����յ��ǿ����Եĸ��Σ�ij����Ʒ����Ҫ�ɷ�Ϊ̼��ƣ���ÿ����Ƭ��1g̼��ƣ���Ԫ��________g��̼����dz�������Ʒ�и�Ԫ������������ߵģ����������������õĹ����������Ĵ�����θ�ᣬд���йصĻ�ѧ����ʽ________��Ϊ�˸��õ����ո�Ԫ�أ�θ��ȱ�����ˣ����øò���Ʒ�����ʱ������________�����ǰ�������������С����������ּ������ɣ�________��
0.4 CaCO3+2HCl=CaCl2+H2O+CO2�� ���� ʳ�����θ���ܴ̼�θ���ڴ���θ�ᣬ������̼��Ƶ��ܽ⣬���ױ���������
����������̼����и�Ԫ�ص������������з�����
����̼��ƻ������ᷴӦ���ɶ�����̼���з�����
����ʳ�����θ�ڻ�̼�θ����θ����з�����
���ÿ����Ƭ�к�1g��̼��ƣ���̼����и�Ԫ�ص�����������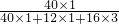 ��100%=40%�����Ը�Ԫ�ص�����Ϊ1g��40%=0.4g���ʴ�Ϊ��0.4g��
��100%=40%�����Ը�Ԫ�ص�����Ϊ1g��40%=0.4g���ʴ�Ϊ��0.4g��
̼��ƺ����ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼���ʴ�Ϊ��CaCO3+2HCl=CaCl2+H2O+CO2����
θ��ȱ��������ʳ�����θ�к�ʳ���̼�Ϊ����������θ�ᣬ�ʴ�Ϊ�����У�ʳ�����θ���ܴ̼�θ���ڴ���θ�ᣬ������̼��Ƶ��ܽ⣬���ױ��������գ�
�������ڽ�����ʽ����д��ʱ������ȷ����Ӧԭ����Ȼ��������ԭ���ҳ���Ӧ�������ͷ�Ӧ���������ݷ���ʽ����д������д����ʽ��
����������̼����и�Ԫ�ص������������з�����
����̼��ƻ������ᷴӦ���ɶ�����̼���з�����
����ʳ�����θ�ڻ�̼�θ����θ����з�����
���ÿ����Ƭ�к�1g��̼��ƣ���̼����и�Ԫ�ص�����������
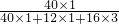 ��100%=40%�����Ը�Ԫ�ص�����Ϊ1g��40%=0.4g���ʴ�Ϊ��0.4g��
��100%=40%�����Ը�Ԫ�ص�����Ϊ1g��40%=0.4g���ʴ�Ϊ��0.4g��̼��ƺ����ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼���ʴ�Ϊ��CaCO3+2HCl=CaCl2+H2O+CO2����
θ��ȱ��������ʳ�����θ�к�ʳ���̼�Ϊ����������θ�ᣬ�ʴ�Ϊ�����У�ʳ�����θ���ܴ̼�θ���ڴ���θ�ᣬ������̼��Ƶ��ܽ⣬���ױ��������գ�
�������ڽ�����ʽ����д��ʱ������ȷ����Ӧԭ����Ȼ��������ԭ���ҳ���Ӧ�������ͷ�Ӧ���������ݷ���ʽ����д������д����ʽ��

��ϰ��ϵ�д�
 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д�
�����Ŀ