��Ŀ����
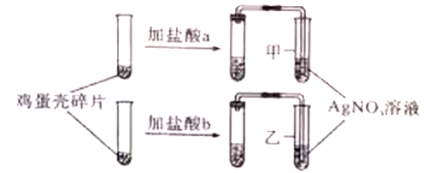
����Ŀ��ij��ѧ�ۺ�ʵ���С���ڽ��С�������Ʒ���о�ʱ��������Ʒ��ǩ���гɷּ�������ʵ���Ƿ���������ȡ������ij�֡������Ʒ���ɷ�˵����ͼ�������������ʽ����Ȼ��ƣ�ȷ��ȡ��Ʒ10.9�ˣ������������Ȼ�����Һʹ����ȫ��Ӧ���䷴Ӧʽ��Na2CO3+CaCl2=2NaCl+CaCO3��������Ӧ���ɵij����ᆳ���ˣ�ϴ�ӣ���ɣ������õ�CaCO3��ɫ����10�ˣ�����������¼��㣺

��1������10.9����Ʒ�д���Na2CO3��������
��2��������Ʒ�к�����Na2CO3������������
���𰸡���1��10.9����Ʒ�д��������Ϊ10.6g��
��2����Ʒ�к��������������Ϊ97.2%��
��������
���������̼���ƺ��Ȼ��Ʒ�Ӧ����̼��ƺ��Ȼ��ƣ�̼��Ʋ�����ˮ���Ȼ�������ˮ�����Ծ������ˣ�ϴ�ӣ���ɵİ�ɫ������̼��ƣ���̼��Ƶ�������10g�����û�ѧ����ʽ���������ݼ����̼���Ƶ�������Ȼ�������Ʒ��̼���Ƶ������������ɣ�
�⣺̼���ƺ��Ȼ��Ʒ�Ӧ����̼��ƺ��Ȼ��ƣ�̼��Ʋ�����ˮ���Ȼ�������ˮ�����Ծ������ˣ�ϴ�ӣ���ɵİ�ɫ������̼��ƣ���̼��Ƶ�������10g��������10��̼�����Ҫ̼���Ƶ�����Ϊx��
Na2CO3+CaCl2=2NaCl+CaCO3��
106 100
x 10g
![]()
x=10.6g
��Ʒ�к��������������=![]() ��100%��97.2%
��100%��97.2%
�𰸣�
��1��10.9����Ʒ�д��������Ϊ10.6g��
��2����Ʒ�к��������������Ϊ97.2%��

����Ŀ����ȥ������������е����ʣ������Լ��ͷ��������е���
ѡ�� | ���� | ���ʣ������� | ���������Լ�����Ҫ���� |
A | O2 | ˮ���� | ͨ��ʢ����ʯ�ҵĸ���� |
B | CaO��ĩ | CaC03 | �������� |
C | KNO3���� | NaCl���� | �ܽ⣬�����ᾧ����� |
D | CuS04��Һ | ���� | �������CuO��ĩ�����ȣ���ַ�Ӧ����� |
A. A B. B C. C D. D