��Ŀ����
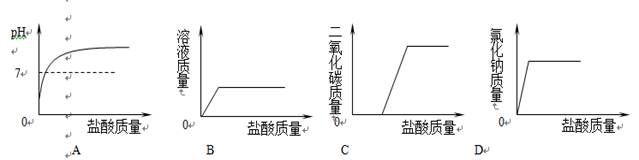
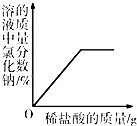
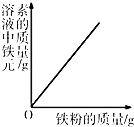
��ʵ���ң�С����25mL�����Ƶ�ϡ��Һ��ϡ��������кͷ�Ӧʵ�飮�������һ������������⣺
��1���������к�ʵ�����ʹ�õ������У��ձ�����������______��______��
��2����������Һ��ϡ������ʱ�����������Ե������ڵμ�ϡ����ǰ��������������Һ�еμ�1��2��______ָʾ����д���壩����ǡ���к�ʱ���۲쵽______��
��3���û�ѧ����ʽ��ʾ�������������ԭ��______��
��4�����кͿ�������Һ���μӵ�ϡ��������������С��������ֲ�ͬ���ͷ���֤����Һ�ﺬ����������õĻ�ѧҩƷ������
������һ��______��
����������______��
����������______��
��1���������к�ʵ�����ʹ�õ������У��ձ�����������______��______��
��2����������Һ��ϡ������ʱ�����������Ե������ڵμ�ϡ����ǰ��������������Һ�еμ�1��2��______ָʾ����д���壩����ǡ���к�ʱ���۲쵽______��
��3���û�ѧ����ʽ��ʾ�������������ԭ��______��
��4�����кͿ�������Һ���μӵ�ϡ��������������С��������ֲ�ͬ���ͷ���֤����Һ�ﺬ����������õĻ�ѧҩƷ������
������һ��______��
����������______��
����������______��
��1������Һ�巢���ķ�Ӧ��Ҫ���ձ��н��У�15mL������������Ҫ��ȡװ��-��Ͳ����Һʱ��У��װ��-��ͷ�ιܣ�
��2����̪������ɫ�������ɫ������Ӧ�����������Һ�еμӷ�̪��Һ������ɫ�պ��ɺ�ɫ��Ϊ��ɫʱ��˵���������ƺ�����ǡ���кͣ�
��3�����������������Һ��Ӧ�����������ƺ�ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��2NaOH+H2SO4�TNa2SO4+2H2O��
��4��������Ժͻ��ý�����Ӧ������ʹʯ����Һ��죬����ʹ̼������Һ��Ӧ�������壬���������Ʋ�������Щ���ʣ����Կ��Ծݴ����ʵ�鷽��
Ϊ��������һ����п�����۲��Ƿ������ݣ�
������������ʯ����Һ���۲��Ƿ��죻
������������̼���ƣ��۲��Ƿ������ݣ�
�ʴ�Ϊ����1����Ͳ���ιܣ�
��2����̪���ɺ����ɫ��
��3��2NaOH+H2SO4�TNa2SO4+2H2O��
��4��������һ����п�����۲��Ƿ������ݣ�
������������ʯ����Һ���۲��Ƿ��죻
������������̼���ƣ��۲��Ƿ������ݣ�
��2����̪������ɫ�������ɫ������Ӧ�����������Һ�еμӷ�̪��Һ������ɫ�պ��ɺ�ɫ��Ϊ��ɫʱ��˵���������ƺ�����ǡ���кͣ�
��3�����������������Һ��Ӧ�����������ƺ�ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��2NaOH+H2SO4�TNa2SO4+2H2O��
��4��������Ժͻ��ý�����Ӧ������ʹʯ����Һ��죬����ʹ̼������Һ��Ӧ�������壬���������Ʋ�������Щ���ʣ����Կ��Ծݴ����ʵ�鷽��
Ϊ��������һ����п�����۲��Ƿ������ݣ�
������������ʯ����Һ���۲��Ƿ��죻
������������̼���ƣ��۲��Ƿ������ݣ�
�ʴ�Ϊ����1����Ͳ���ιܣ�
��2����̪���ɺ����ɫ��
��3��2NaOH+H2SO4�TNa2SO4+2H2O��
��4��������һ����п�����۲��Ƿ������ݣ�
������������ʯ����Һ���۲��Ƿ��죻
������������̼���ƣ��۲��Ƿ������ݣ�

��ϰ��ϵ�д�
�����Ŀ