��Ŀ����
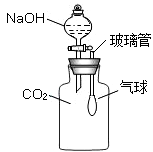
��ѧʵ���ᳫ��ɫ��������ʵ��װ�ý����ͻ��Ľ���һ���ܺõ�;����ͼ����ʵ������ȡ������CO2��װ�ã�ͼ���Ƕ�ͼ��ʵ��װ�õġ��͡����Ľ����װ�ã�

��1��ͼ���������ϵι���ʵ���е�������ͼ���е�������ͬ��_________��������ĸ��ţ���
��2��ͨ���ü�װ����ɸ�ʵ����Ҫ�������ǡ��͡�ʵ��װ��������10�������á��͡�ʵ��װ�þ��е��ŵ�����_________����
��3��д��ʵ������ȡ������̼�Ļ�ѧ����ʽ��_________����
��4��ľ̿������ͭ�ڸ����¿ɷ�����ѧ��ӦC+CuO�TC+CO2�����д����ȷ�Ļ�ѧ����ʽ��_________����ʵ����Ϊʲô���ô˷�����ȡ������̼��ԭ����_________����

��1��ͼ���������ϵι���ʵ���е�������ͼ���е�������ͬ��_________��������ĸ��ţ���
��2��ͨ���ü�װ����ɸ�ʵ����Ҫ�������ǡ��͡�ʵ��װ��������10�������á��͡�ʵ��װ�þ��е��ŵ�����_________����
��3��д��ʵ������ȡ������̼�Ļ�ѧ����ʽ��_________����
��4��ľ̿������ͭ�ڸ����¿ɷ�����ѧ��ӦC+CuO�TC+CO2�����д����ȷ�Ļ�ѧ����ʽ��_________����ʵ����Ϊʲô���ô˷�����ȡ������̼��ԭ����_________����
��1��A ��2��ҩƷ�����٣������ķ�����Ҳ�٣����������
��3��CaC03+2HCl=CaCl2+H20+C02��
��4��C+2CuO 2Cu+CO2������Ӧ���ڸ����½��У��������㣻��ȡ�Ķ�����̼�л����һ����̼��
2Cu+CO2������Ӧ���ڸ����½��У��������㣻��ȡ�Ķ�����̼�л����һ����̼��
��3��CaC03+2HCl=CaCl2+H20+C02��
��4��C+2CuO
 2Cu+CO2������Ӧ���ڸ����½��У��������㣻��ȡ�Ķ�����̼�л����һ����̼��
2Cu+CO2������Ӧ���ڸ����½��У��������㣻��ȡ�Ķ�����̼�л����һ����̼�������������1��ͼ���������ϵι���ʵ���е�������ͼ���еķ�Һ©��������ͬ�����A��
��2�����á��͡�ʵ��װ�þ��е��ŵ���ҩƷ�����٣������ķ�����Ҳ�٣�������������ҩƷ�����٣������ķ�����Ҳ�٣����������
��3������ʵ�����ô���ʯ��ϡ���ᷴӦ��ȡ������̼�ķ�Ӧ��������Ӧ����д����ѧ��Ӧ�ķ���ʽΪ��CaC03+2HCl=CaCl2+H20+C02����
��4����Ӧ���ڸ����½��У������������������̼��̼�ڸ���������������һ����̼����Ӧ�Ļ�ѧ����ʽΪ��CO2��C
 2CO��������ȡ�Ķ�����̼�л����һ����̼�����Ӧ���ڸ����½��У��������㣻��ȡ�Ķ�����̼�л����һ����̼��
2CO��������ȡ�Ķ�����̼�л����һ����̼�����Ӧ���ڸ����½��У��������㣻��ȡ�Ķ�����̼�л����һ����̼��
��ϰ��ϵ�д�
�����Ŀ