��Ŀ����
 ��2011?��ɽ��Ϊ���о������غ㶨�ɣ��������ͼ������ȼ��ǰ�������ⶨ����ʵ�飬������й����⣺
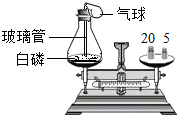
��2011?��ɽ��Ϊ���о������غ㶨�ɣ��������ͼ������ȼ��ǰ�������ⶨ����ʵ�飬������й����⣺��1��װ�ã���ƿ�ĵײ�����һ��ϸɳ����������
��ֹ��ƿ�ײ��ֲ����ȱ���
��ֹ��ƿ�ײ��ֲ����ȱ���
����2��ȼ��ǰ��������ƿ��������Ϊ27.6g����ͼ��������ƽ������Ķ���Ϊ
2.6
2.6
g����3������ȼ�գ�����ȼ�չ����п�����������
������ɫ�̣������ʹ�����С
������ɫ�̣������ʹ�����С
����4��ȼ�պ����������������ƽָ��ƫ���ұߣ���ɵ�ԭ�������
װ��©��������ƿδ��ȴ�ȣ�
װ��©��������ƿδ��ȴ�ȣ�
��5����˼������ȼ��
����
����
������ء������ء��������غ㶨�ɣ��������μӷ�Ӧ�ĸ����ʵ������ܺ������ɵĸ����ʵ������ܺ����
�μӷ�Ӧ�ĸ����ʵ������ܺ������ɵĸ����ʵ������ܺ����
����������1������ȼ�����ɵ������������¶Ⱥܸߣ������䣻
��2������������ƽ����Ϊ�����������������������
��3�����ݰ���ȼ�յ�����ش𣮰�������ȼ��ڿ����о���ȼ�գ������������̣����������������Ĺ���С������
��4��ȼ�պ����������������ƽָ��ƫ���ұߣ����ұ�ƫ�أ��ʿ��Ʋ⣻
��5���ڻ�ѧ�仯����ѭ�����غ㶨�ɣ��ʿ��Ʋ����ɣ�
��2������������ƽ����Ϊ�����������������������
��3�����ݰ���ȼ�յ�����ش𣮰�������ȼ��ڿ����о���ȼ�գ������������̣����������������Ĺ���С������
��4��ȼ�պ����������������ƽָ��ƫ���ұߣ����ұ�ƫ�أ��ʿ��Ʋ⣻
��5���ڻ�ѧ�仯����ѭ�����غ㶨�ɣ��ʿ��Ʋ����ɣ�
����⣺��1����ƿӦԤ��װ������ϸɰ��ԭ���Ƿ�ֹ��ȼ�����ɵ�������������ը����ƿ����װ�ã���ƿ�ĵײ�����һ��ϸɳ���������Ƿ�ֹ��ƿ�ײ��ֲ����ȱ��ѣ�
��2��ȼ��ǰ��������ƿ��������Ϊ27.6g������ͼ������ƽ������Ķ���Ϊ27.6-��20+5��=2.6g��
��3��������ȼ��ȼ�պ��������������ף������������ǰ�ɫ���壬����ʱ��С��������ʽ���֣�����ȥΪ�����̡����ʴ�Ϊ��ȼ�վ��ң����ɴ������̣�
��4��ȼ�պ����������������ƽָ��ƫ���ұߣ����ұ�ƫ�أ�����ɵ�ԭ�������װ��©��������ƿδ��ȴ�ȣ���
��5����ѧ�仯����ѭ�����غ㶨�ɣ���˼������ȼ�����������غ㶨�ɣ������Dzμӷ�Ӧ�ĸ����ʵ������ܺ������ɵĸ����ʵ������ܺ���ȣ�
�ʴ�Ϊ����1����ֹ��ƿ�ײ��ֲ����ȱ��ѣ�
��2��2.6��
��3��������ɫ�̣������ʹ�����С��
��4��װ��©��������ƿδ��ȴ�ȣ���
��5�����أ��μӷ�Ӧ�ĸ����ʵ������ܺ������ɵĸ����ʵ������ܺ���ȣ�
��2��ȼ��ǰ��������ƿ��������Ϊ27.6g������ͼ������ƽ������Ķ���Ϊ27.6-��20+5��=2.6g��
��3��������ȼ��ȼ�պ��������������ף������������ǰ�ɫ���壬����ʱ��С��������ʽ���֣�����ȥΪ�����̡����ʴ�Ϊ��ȼ�վ��ң����ɴ������̣�
��4��ȼ�պ����������������ƽָ��ƫ���ұߣ����ұ�ƫ�أ�����ɵ�ԭ�������װ��©��������ƿδ��ȴ�ȣ���
��5����ѧ�仯����ѭ�����غ㶨�ɣ���˼������ȼ�����������غ㶨�ɣ������Dzμӷ�Ӧ�ĸ����ʵ������ܺ������ɵĸ����ʵ������ܺ���ȣ�
�ʴ�Ϊ����1����ֹ��ƿ�ײ��ֲ����ȱ��ѣ�
��2��2.6��
��3��������ɫ�̣������ʹ�����С��
��4��װ��©��������ƿδ��ȴ�ȣ���
��5�����أ��μӷ�Ӧ�ĸ����ʵ������ܺ������ɵĸ����ʵ������ܺ���ȣ�
�������ڻ�ѧ��Ӧ����ѭ�����غ㶨�ɣ��μӷ�Ӧ�����ʵ��������ڷ�Ӧ�����ɵ����ʵ���������������Ϊ�����������غ㶨�ɵ�̽����ּ�ڿ��鲻�淶������ʵ������Ӱ�죬����ʱҪ��ȷ�����ڵķ�Ӧ����������������仯���Ӷ��ó���ȷ���ۣ�

��ϰ��ϵ�д�
 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
�����Ŀ