��Ŀ����
��������������ع����ڲ�ͬ�¶�ʱ���ܽ�ȡ�
| �¶�/�� | 0 | 20 | 40 | 60 | 80 |
| �ܽ��/g | 13.3 | 31.6 | 63.9 | 110 | 169 |
��2��20��ʱ����100 gˮ�м���31.6 g����أ�����ܽ��õ� ������͡������͡�����Һ��
��3��20��ʱ����100 gˮ�м���40 g����أ���ʹ�������ȫ�ܽ⣬�ɲ��õķ����� ��
��4����ͼ��С�ձ���ʢ�ŵ��ǣ�2�������õ��������Һ�������������������ʷֱ�С�ĵؼ��뵽���ձ���ˮ�У����Ͻ��裬һ���ܹ�ʹС�ձ����й����������� ������ĸ��
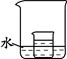
A���� B��Ũ���� C������� D���ɱ� E���������� F��������
��5����һ�������͵�ʯ��ˮ�м�����������ʯ�һָ���ԭ�¶ȣ������������� �����иı������ ������ʾ��CaO+H2O��Ca(OH)2 ��
A�����ʵ����� B���ܼ������� C����Һ������ D�����ʵ���������
��1��ˮ
��2������
��3����ˮ������
��4��A C D
��5�������ʯ��ˮ����� A B C
����

��ϰ��ϵ�д�
�����Ŀ


 28����������������ع����ڲ�ͬ�¶�ʱ���ܽ�ȣ�
28����������������ع����ڲ�ͬ�¶�ʱ���ܽ�ȣ� ��������������ع����ڲ�ͬ�¶�ʱ���ܽ�ȣ�
��������������ع����ڲ�ͬ�¶�ʱ���ܽ�ȣ�