��Ŀ����
 С�����ּ���ʩ���õ�̼����泥�NH4HCO3�������ˣ����ŵ���һ�ɴ̼��Ե���ζ�����ܺ��棬���Ǻ�ͬѧ�ǽ���̽��������һͬ���룺
С�����ּ���ʩ���õ�̼����泥�NH4HCO3�������ˣ����ŵ���һ�ɴ̼��Ե���ζ�����ܺ��棬���Ǻ�ͬѧ�ǽ���̽��������һͬ���룺
[�������]̼����識��ٵ�ԭ����ʲô��
[�������]̼����������ֽ⣬�������Ϊˮ��������̼��������
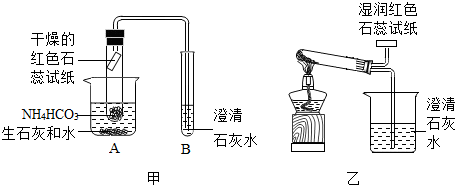
[ʵ�����]С���������ͼ��ʾ��װ�üף�С�������װ���ҽ���ʵ�飨ʵ��װ���е�����̨��ʡ�ԣ���
��1��С����������______��֤��ʵ���������ˮ�Ͱ�����
��2������B�г���ʯ��ˮ______��֤���������ж�����̼���ɣ�
[ʵ�����]̼����������ֽ⣬������ˮ��������̼��������
[ʵ������]������ͬѧ�ǵ�̽����У������______װ�ò���ѧ����װ�õ�������______����һ��װ�õ���Խ�Ի�����Щ______��
[ʵ�鷴˼]��������ʵ�飬����Ϊ����̼������ڱ���ʱӦע���������______��
[��չ̽��]��1��С��ͬѧ��Ϊ����װ��B�г���ʯ��ˮ��ΪNaOH��Һ���پ���ijʵ�������Ҳ����֤��̼����立ֽ���ж�����̼���ɣ����������Ʋ����ʵ�飺
| ʵ�鲽�� | ʵ������ | ��Ӧ�Ļ�ѧ����ʽ |
______��
��2����֤������̼������ʹ����ʯ��ˮ����ǵ����ʣ��ʴ𰸣������
��ʵ�����ۡ�ʹ��ʪ���ɫʯ����ֽ��ȷ���������Ƿ���ˮ������������װ�ò���ѧ��װ�ü�����ʯ�Һ�ˮ��Ӧ�ų���������Ϊ��Դ������ƾ��ƣ���Լ��Դ����������Ⱦ���ʴ𰸣��ң�ʹ��ʪ���ɫʯ����ֽ��ȷ���������Ƿ���ˮ����������ʯ�Һ�ˮ��Ӧ�ų���������Ϊ��Դ������ƾ��ƣ���Լ��Դ����������Ⱦ��
��ʵ�鷴˼����Ϊ̼������ֽ⣬����Ӧ�ܷⱣ�棬�������������ʴ𰸣��ܷⱣ�棬����������
[��չ̽��]������ʯ��ˮ�����������ƶ�����̼������Ӧ����̼���ƣ�̼�����������ᷴӦ�����Ȼ��ƺͶ�����̼����ˮ���ʴ𰸣�
| ʵ�鲽�� | ʵ������ | ��Ӧ�Ļ�ѧ����ʽ |
| ȡװ��B����Һ�������μ�ϡ���� | �������� | Na2CO3+2HCl=2NaCl+H2O+CO2�� |
����������ͼ��ʵ�飬�����ʯ����ֽ������ɫ֤����ˮ�Ͱ������ɣ�����ʯ��ˮ�����֤���ж�����̼���ɣ���֤������̼�ó���ʯ��ˮ��������������ѡ�����淽����ע�����
���������⿼����̼����淋����ʵ�̽�����ۺ���ǿ��

��11�֣�С�����ּ���ʩ���õ�̼����泥�NH4HCO3�������ˣ����ŵ���һ�ɴ̼��Ե���ζ�����ܺ��棬���Ǻ�ͬѧ�ǽ���̽��������һͬ���룺
[�������]̼����識��ٵ�ԭ����ʲô��
[�������]̼����������ֽ⣬�������Ϊˮ��������̼��������
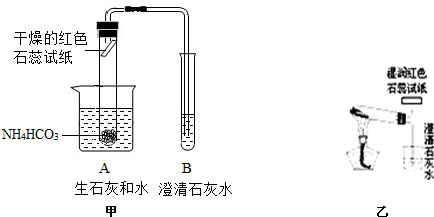
[ʵ�����]С���������ͼ��ʾ��װ�üף�С�������װ���ҽ���ʵ�飨ʵ��װ���е�����̨��ʡ�ԣ���


�� ��
��1��С���������� ��֤��ʵ���������ˮ�Ͱ�����
��2������B�г���ʯ��ˮ ��֤���������ж�����̼���ɣ�
[ʵ�����]̼����������ֽ⣬������ˮ��������̼��������
[ʵ������]������ͬѧ�ǵ�̽����У������ װ�ò���ѧ����װ�õ������� ����һ��װ�õ���Խ�Ի�����Щ ��
[ʵ�鷴˼]��������ʵ�飬����Ϊ����̼������ڱ���ʱӦע��������� ��
[��չ̽��]��1��С��ͬѧ��Ϊ����װ��B�г���ʯ��ˮ��ΪNaOH��Һ���پ���ijʵ�������Ҳ����֤��̼����立ֽ���ж�����̼���ɣ����������Ʋ����ʵ�飺
| ʵ�鲽�� | ʵ������ | ��Ӧ�Ļ�ѧ����ʽ |
|
|
|
|
��2���������Ϸ������㻹������ƺ���ʵ�鷽����Ҫ����ѡ�õ������루1�����ڲ�ͬ���
��11�֣�С�����ּ���ʩ���õ�̼����泥�NH4HCO3�������ˣ����ŵ���һ�ɴ̼��Ե���ζ�����ܺ��棬���Ǻ�ͬѧ�ǽ���̽��������һͬ���룺
[�������]̼����識��ٵ�ԭ����ʲô��
[�������]̼����������ֽ⣬�������Ϊˮ��������̼��������
[ʵ�����]С���������ͼ��ʾ��װ�üף�С�������װ���ҽ���ʵ�飨ʵ��װ���е�����̨��ʡ�ԣ���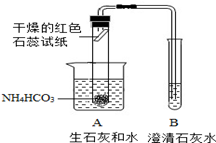

�� ��
��1��С���������� ��֤��ʵ���������ˮ�Ͱ�����
��2������B�г���ʯ��ˮ ��֤���������ж�����̼���ɣ�
[ʵ�����]̼����������ֽ⣬������ˮ��������̼��������
[ʵ������]������ͬѧ�ǵ�̽����У������ װ�ò���ѧ����װ�õ������� ����һ��װ�õ���Խ�Ի�����Щ ��
[ʵ�鷴˼]��������ʵ�飬����Ϊ����̼������ڱ���ʱӦע��������� ��
[��չ̽��]��1��С��ͬѧ��Ϊ����װ��B�г���ʯ��ˮ��ΪNaOH��Һ���پ���ijʵ�������Ҳ����֤��̼����立ֽ���ж�����̼���ɣ����������Ʋ����ʵ�飺
| ʵ�鲽�� | ʵ������ | ��Ӧ�Ļ�ѧ����ʽ |
| | | |