��Ŀ����
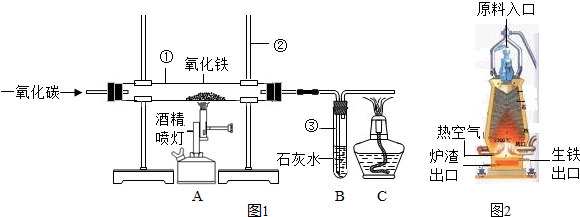
��ͼ1��ʵ����ģ��������װ��ͼ���Իش�
��1�������ٵ�������______�������ڵ�������______��
��2��ʵ������������з�����Ӧ�Ļ�ѧ����ʽ��______���������е�������______��
��3��ʵ������в�����β������ֱ���ŷŵ�ԭ����______��
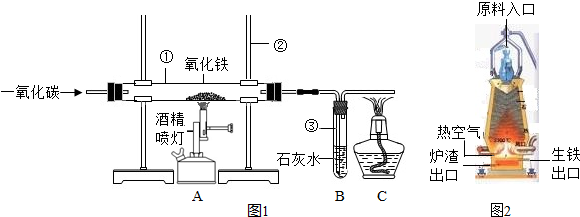
��4��ͼ2�ǹ�ҵ������������¯�Ľṹͼ��ʵ��������������ԭ������ʯ����̿��ʯ��ʯ�Ǵ�ԭ����ڼ��룬���н�̿�ڸ�¯����ʱ������֮һ�Dz������£���һ����Ϊ______���������ڵ���¯�����ڵ�ԭ����______��
��5��ȡ������¯�����������������ձ��У���������ϡ���ᣬ�ɹ۲쵽��������______��������Ӧ�Ļ�ѧ����ʽΪ______������Ӧֹͣ���ɹ۲쵽�ձ��ײ��к�ɫ�������������Ҫ�ǣ��ѧʽ��______��
��1�������ٵ�������______�������ڵ�������______��
��2��ʵ������������з�����Ӧ�Ļ�ѧ����ʽ��______���������е�������______��
��3��ʵ������в�����β������ֱ���ŷŵ�ԭ����______��
��4��ͼ2�ǹ�ҵ������������¯�Ľṹͼ��ʵ��������������ԭ������ʯ����̿��ʯ��ʯ�Ǵ�ԭ����ڼ��룬���н�̿�ڸ�¯����ʱ������֮һ�Dz������£���һ����Ϊ______���������ڵ���¯�����ڵ�ԭ����______��
��5��ȡ������¯�����������������ձ��У���������ϡ���ᣬ�ɹ۲쵽��������______��������Ӧ�Ļ�ѧ����ʽΪ______������Ӧֹͣ���ɹ۲쵽�ձ��ײ��к�ɫ�������������Ҫ�ǣ��ѧʽ��______��

��1������ʵ���ҳ������������ƿ�֪�������ٵ������� �����ܣ������ڵ������� ����̨��
��2��һ����̼��ԭ��������ͬʱ���ɶ�����̼�������������з�����Ӧ�Ļ�ѧ����ʽΪ��Fe2O3+3CO�T2Fe+3CO2��
������̼��ʹ����ʯ��ˮ����ǣ������������е������� ����ǣ�
��3����Ϊһ����̼�ж�������ʵ������в�����β������ֱ���ŷţ���Ⱦ������
��4������Ҫ�õ���̿�������������ã�������Ҫ���£�һ���潹̿��ȼ�յĹ������ܷų��������ȣ���һ������������Ҫһ����̼����̿�ֿ��Ѹ����ɵĶ�����̼��ԭΪһ����̼���������ڵ���¯�����ڵ�ԭ���ǣ������ܶȴ���¯���ܶȣ�
��5����¯�����������������ձ��У���������ϡ����ķ�Ӧ����ʽΪ��Fe+2HCl=FeCl2+H2������Ӧ�������������ݣ���Һ��Ϊdz��ɫ����Ӧֹͣ�۲쵽�ձ��ײ��к�ɫ�������������Ҫ��̼��C��
�ʴ�Ϊ����1���ٲ����ܣ� ������̨��
��2��Fe2O3+3CO�T2Fe+3CO2������ǣ�
��3��β���е�һ����̼��Ⱦ������
��4����ԭ�����������ܶȴ���¯����
��5�������������ݣ���Һ��Ϊdz��ɫ�� Fe+2HCl=FeCl2+H2����C��
��2��һ����̼��ԭ��������ͬʱ���ɶ�����̼�������������з�����Ӧ�Ļ�ѧ����ʽΪ��Fe2O3+3CO�T2Fe+3CO2��
������̼��ʹ����ʯ��ˮ����ǣ������������е������� ����ǣ�
��3����Ϊһ����̼�ж�������ʵ������в�����β������ֱ���ŷţ���Ⱦ������
��4������Ҫ�õ���̿�������������ã�������Ҫ���£�һ���潹̿��ȼ�յĹ������ܷų��������ȣ���һ������������Ҫһ����̼����̿�ֿ��Ѹ����ɵĶ�����̼��ԭΪһ����̼���������ڵ���¯�����ڵ�ԭ���ǣ������ܶȴ���¯���ܶȣ�
��5����¯�����������������ձ��У���������ϡ����ķ�Ӧ����ʽΪ��Fe+2HCl=FeCl2+H2������Ӧ�������������ݣ���Һ��Ϊdz��ɫ����Ӧֹͣ�۲쵽�ձ��ײ��к�ɫ�������������Ҫ��̼��C��
�ʴ�Ϊ����1���ٲ����ܣ� ������̨��
��2��Fe2O3+3CO�T2Fe+3CO2������ǣ�
��3��β���е�һ����̼��Ⱦ������
��4����ԭ�����������ܶȴ���¯����
��5�������������ݣ���Һ��Ϊdz��ɫ�� Fe+2HCl=FeCl2+H2����C��

��ϰ��ϵ�д�
 ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д� Сѧ��ʱ��ҵȫͨ����ϵ�д�
Сѧ��ʱ��ҵȫͨ����ϵ�д�
�����Ŀ