��Ŀ����
���û�ѧ����ʽ���Դ����ķ����о����ʵı仯�������ϡ�����þ��Ӧ���ⶨþ�����ԭ����������þ�����ԭ������ΪX����ͼ��ijͬѧ��ƵIJⶨþ��ԭ��������װ�ã�ʢ��þ�������ϸ�������С�ף���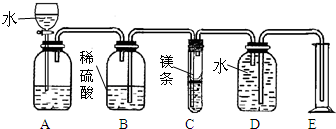
��1��þ��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ
��2�����Ӻ�������Ҫ���еIJ�����Ҫ�����¼������ٴ�����C�е����ʻָ�������ʱ���������E��ˮ�����ΪVmL����������Ϊm1g������ƽ�ϳ�ȡ����Ϊm2g��þ����������Ͷ���Թ�C�У��ۼ��װ�õ������ԣ�������A�Ϸ�Һ©���Ļ�������þ����ȫ�ܽ�ʱ���ٹرջ��������������������Ⱥ�˳����
��3���ɣ�2����ʵ�����ݣ�þ�����ԭ������X�ļ���ʽΪ
��
��4����δ���Թ�C��ȴ�����¾Ͷ�ȡE��ˮ��������⽫��ʹ���þ�����ԭ���������ݣ��ƫ�ߡ���ƫ�͡�����Ӱ�족��

��1��þ��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ
Mg+H2SO4=MgSO4+H2��
Mg+H2SO4=MgSO4+H2��
����2�����Ӻ�������Ҫ���еIJ�����Ҫ�����¼������ٴ�����C�е����ʻָ�������ʱ���������E��ˮ�����ΪVmL����������Ϊm1g������ƽ�ϳ�ȡ����Ϊm2g��þ����������Ͷ���Թ�C�У��ۼ��װ�õ������ԣ�������A�Ϸ�Һ©���Ļ�������þ����ȫ�ܽ�ʱ���ٹرջ��������������������Ⱥ�˳����
�ۢڢܢ�
�ۢڢܢ�
����A�м���ˮ��Ŀ������B�е�ϡ����ѹ��C����þ����Ӧ
��B�е�ϡ����ѹ��C����þ����Ӧ
����3���ɣ�2����ʵ�����ݣ�þ�����ԭ������X�ļ���ʽΪ
| 2m2g |
| m1 |
| 2m2g |
| m1 |
��4����δ���Թ�C��ȴ�����¾Ͷ�ȡE��ˮ��������⽫��ʹ���þ�����ԭ���������ݣ��ƫ�ߡ���ƫ�͡�����Ӱ�족��
ƫ��
ƫ��
������������ⶨþ����Է��������ķ�����ͨ��þ�����ᷴӦ���ⶨ���ɵ��������������ٸ��ݻ�ѧ����ʽ�����þ����Է���������Ҫ��ȷÿ��װ�õ����ã�Aװ����һ����ѹ���ã���Cװ����þ����������з�Ӧ��Eװ�ò���ˮ�������Ҳ�������ɵ������ſ�ˮ�������Eװ���ǰ��������������ת����ˮ�����������ʵ��ԭ����װ�õ����ã����ǿ�����������װ�ã�Ϊ��ʹʵ����ȷ����������������������õ�þ����������Ҫȷ���ݴ˷�����ɣ�
����⣺��1������þ��ϡ���ᷴӦʱ����������þ���������䷽��ʽΪ��Mg+H2SO4=MgSO4+H2��
��2������ʵ���Ŀ�ĺͽ���ʵ���Ҫ���֪��ʵ��IJ��������ǣ����Ӻ�������Ҫ�ȼ�������ԣ��ڶ�þ���������в������ٷ���ҩƷ�����з�Ӧ��Ȼ���ռ�����������������
װ��A���������Ʒ�Ӧ�����ģ���Һ©����ʢ�ŵ�Һ����ˮ����������ʹAƿ�е��������Eƿ������Eƿ�е���ѹ����ϡ����ѹ��Bװ����Mg������ѧ��Ӧ��
��3����þ�����ԭ��������x
Mg+H2SO4=MgSO4+H2��
x 2
m2g m1g
=
x=
��4����δ���Թ�C��ȴ�����¾Ͳ�����ͲE��ˮ�������E�е�ˮ�����Ҫ��ʵ�������������ɵ�������ļ����⽫��ʹ����þ�����ԭ����������ƫ�ͣ�
�ʴ�Ϊ����1��Mg+H2SO4=MgSO4+H2����
��2���ۢڢܢ٣���B�е�ϡ����ѹ��C����þ����Ӧ
��3��
�� ƫ�ͣ�
��2������ʵ���Ŀ�ĺͽ���ʵ���Ҫ���֪��ʵ��IJ��������ǣ����Ӻ�������Ҫ�ȼ�������ԣ��ڶ�þ���������в������ٷ���ҩƷ�����з�Ӧ��Ȼ���ռ�����������������
װ��A���������Ʒ�Ӧ�����ģ���Һ©����ʢ�ŵ�Һ����ˮ����������ʹAƿ�е��������Eƿ������Eƿ�е���ѹ����ϡ����ѹ��Bװ����Mg������ѧ��Ӧ��
��3����þ�����ԭ��������x
Mg+H2SO4=MgSO4+H2��
x 2
m2g m1g
| x |
| 2 |
| m2g |
| m1g |
x=
| 2m2g |
| m1 |
��4����δ���Թ�C��ȴ�����¾Ͳ�����ͲE��ˮ�������E�е�ˮ�����Ҫ��ʵ�������������ɵ�������ļ����⽫��ʹ����þ�����ԭ����������ƫ�ͣ�
�ʴ�Ϊ����1��Mg+H2SO4=MgSO4+H2����
��2���ۢڢܢ٣���B�е�ϡ����ѹ��C����þ����Ӧ
��3��
| 2m2g |
| m1 |
�����������ۺϿ�����ѧ���Ļ���ʵ���������漰ʵ�������������Ŀ��顢��ѧ��������֪ʶ��ֻ�о��н���ʵ�Ļ�ѧ֪ʶ�ۺ������������ô��⣮

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��2013?������һģ����ǰ��������۵ļ۸����Ԫ���ϰ�Ԫ���ȣ�������ۡ��ָж�û�����Բ��죮
��2013?������һģ����ǰ��������۵ļ۸����Ԫ���ϰ�Ԫ���ȣ�������ۡ��ָж�û�����Բ��죮


