��Ŀ����
�Ҵ����Ը��������ס������Ϊԭ�ϣ������͡�������Ƶã����ڿ�������Դ���������м��������Ҵ���Ϊ����ȼ�ϣ��ɽ�ʡʯ����Դ����������β������Ⱦ���Ҵ���C2H5OH����ȫȼ��ʱ����CO2��H2O��������������㣬�Ҵ�ȼ�տ��ܻ���CO���ɡ�
��1���Ƶ��Ҵ��Ĺ������� �仯����������ѧ����
��2���Ҵ�Ҫȼ�������������������Ӵ��⣬�����������һ��������
��
��3���Ҵ������ƾ������ȼ�ϣ�����ʵ��Ĺ����У��ƾ��Ʋ�����ȼ��������Ӧ��ȡ������ʩ�� ��
��4����������װ�ý���ʵ�飬ȷ֤�Ҵ�ȼ�ղ�������CO��CO2��H2O����ȷ������˳��Ϊȼ�ղ������ ������ ������ ������ ������ ����β��������ע�⣺��Щװ�ÿɲ�ѡ�ã���Щ���ظ�ѡ�ã���

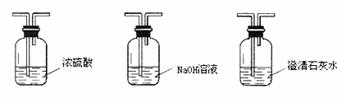
A B C D E
��1�� ��ѧ ��2�� �¶ȴﵽ�Ż�� ��3�� ��ʪĨ������
��4��ȼ�ղ������E������C������B������D������C����β������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�