��Ŀ����
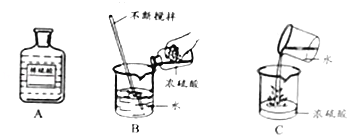
����Ŀ��ij��ȤС���ͬѧ��ʵ���ռ���һͰ����FeSO4��CuSO4�ķ�Һ����������л��ս���ͭ�������������壬��������²�����������Ϸ����ش��������⣺
��1������A�к����� �� ��ɫ����XΪ�����ѧʽ����
��2��������з�Ӧ�Ļ�ѧ����ʽΪ �� �÷�Ӧ�������ֻ��������е���Ӧ��
��3������a������Ϊ �� �ڸò������õ��˲�����������Ϊ ��
��4��������������������������������������������=����ԭ��Һ������������������
���𰸡�
��1������ͭ��H2
��2��Fe+CuSO4�TFeSO4+Cu���û�
��3�����ˣ�����
��4����
���������⣺��1��������м�������ǹ����ģ�����������ͭ��Ӧ��������������ͭ�����Թ���A�к�������ͭ�����������ᷴӦ��������������������������ɫ����XΪH2����2������������ͭ��Ӧ��������������ͭ����ѧ����ʽΪ��Fe+CuSO4�TFeSO4+Cu���÷�Ӧ�����û���Ӧ����3�����˿��Խ������Թ������Һ�з��룬���Բ���a������Ϊ���ˣ��ڸò������õ��˲�����������Ϊ��������4����������ͭ��Ӧ����������������������ϡ���ᷴӦ�����������������������������������������������ԭ��Һ������������������
���Դ��ǣ���1������ͭ��H2����2��Fe+CuSO4�TFeSO4+Cu���û�����3�����ˣ���������4������
�����㾫����ͨ��������ù��˲�����ע������ͽ������ϵ�ѡ�����ݣ����չ��˲���ע�������һ���������͡������������˺���Һ��Ȼ���ǵĿ���ԭ����:�ٳн���Һ���ձ����ɾ����㵹Һ��ʱҺ�������ֽ��Ե����ֽ���𣻺�ɫ������ͨ��ָ�����̡��������ǵĺϽ��ؽ�������ͭ��п��Ǧ�ȣ���ɫ��������������ơ�þ�����ȣ���ɫ������ͨ����ָ����ɫ����������������������Խ����⣮