题目内容
【题目】化学兴趣小组的同学将一包碳酸钠样品溶于75.1g水制成溶液,然后向其中加入溶质质量分数10%的氯化钙溶液至恰好完全反应,得到沉淀10g,将滤液蒸干得到固体纯净物25g。请回答问题:

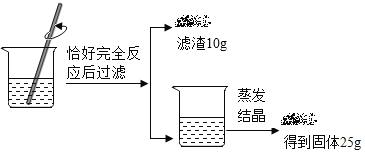
(1)写出发生反应的化学方程式________。
(2)列出根据已知条件求解混合物中碳酸钠质量(x)的比例式________。
(3)碳酸钠样品中其它成分的质量为_________。
(4)反应所得滤液的溶质质量分数为________。
(5)若将239t该混合物中的碳酸钠完全转化为氢氧化钠,可制得氢氧化钠_____t。
【答案】![]()
![]() 13.3g 12.5% 80
13.3g 12.5% 80
【解析】
(1)碳酸钠和氯化钙反应生成碳酸钙沉淀和氯化钠,化学方程式为:Na2CO3+CaCl2=2NaCl+CaCO3↓;
(2)设参加反应的碳酸钠质量为x,生成氯化钠质量为y,消耗氯化钙溶液为z
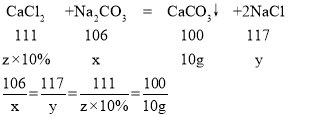
解得x=10.6g,y=11.7g,z=111g
故求解混合物中碳酸钠质量(x)的比例式为![]() ;
;
(3)所以原固体混合物中其它成分的质量为:25g-11.7g=13.3g;
答:原固体混合物中其它成分的质量为13.3g。
(4)反应后所得滤液中溶质的质量分数为:![]() ×100%=12.5%;
×100%=12.5%;
答:反应后所得滤液中溶质的质量分数为12.5%。
(5)239t该混合物中的碳酸钠的质量为239t×![]() ×100%=106t,设生成氢氧化钠的质量为m。
×100%=106t,设生成氢氧化钠的质量为m。
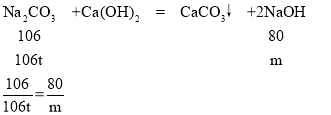
解得m=80t
答:可制得氢氧化钠80t。

练习册系列答案
 阅读快车系列答案
阅读快车系列答案
相关题目