��Ŀ����
 ijУ�о���ѧϰС�������һ����Ȥ��ʵ��̽����
ijУ�о���ѧϰС�������һ����Ȥ��ʵ��̽����������⣺ʵ������һƿ���õ�NaOH�����ʳ̶�������
��Ʒ������ȳ�ȡ21.2g ��NaOH��Ʒ������ΪNa2CO3���������Һ��Ȼ������Һ����μ���һ������������ϡ����ֱ����������������CO2�����������Na2CO3���������Ӷ���һ��ȷ����Ʒ��NaOH������������
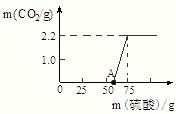
����ʵ�飺ʵ���ü���ϡ��������������CO2�����������ϵ����ͼ��ʾ��
���ݴ�����д�����¼�����̣�
��1������Ʒ��Na2CO3������Ϊ���٣�
��2��ϡ��������������Ƕ��٣�
��3����Ӧ�����ɵ���Һ����������������
������������ͼ������й������̽���������Ƶı��ʳ̶ȣ����ݲ���������̼�������������Ʒ��̼���Ƶ�������ͬЩ���Լ����ԭ��Ʒ���������Ƶ�������Ȼ�����̼���ơ�����������ϡ���ᷴӦ�Ļ�ѧ����ʽ�����ϡ�����е����ʡ����ɵ���Һ�е����ʣ������ɼ������Һ�����ʵ�����������
����⣺��ͼ���֪���ڼ�����Ʒ�е�����ʱ����ϡ���ᣬ��������̼���������ԭ�����ڼ�����Һ�У����������кͷ�Ӧ�����������壻������������������ȫ��Ӧ��ʼ����Ʒ�е�̼���Ʒ�����Ӧ�����ɶ�����̼���壬�������μ�����ʱ�����ղ�������������Ϊ2.2g�����Ҳ��ٱ仯��˵����Ʒ�е�̼������ȫ��Ӧ��ϣ�
�����Ʒ��Na2CO3������Ϊx����Na2CO3��Ӧ��H2SO4������Ϊy����Na2CO3��Ӧ���ɵ������Ƶ�����Ϊz��
Na2CO3+H2SO4�TNa2SO4+H2O+CO2��
106 98 142 44
x y z 2.2g
��1��
=
x=5.3g
=
y=4.9g
=
z=7.1g
��Ʒ���������Ƶ�����Ϊ21.2g-5.3g=15.9g������NaOH��Ӧ��H2SO4������Ϊa����NaOH��Ӧ���ɵ������Ƶ�����Ϊb��
2NaOH+H2SO4�TNa2SO4+H2O
80 98 142
15.9g a b
=
a=19.5g
=
b=28.2g
��2��ϡ���������������
��100%=32.5%
��3����Ӧ�����ɵ���Һ��������������Ϊ
��100%=37.6%��
�𣺣�1������Ʒ��Na2CO3������Ϊ5.3g����2��ϡ���������������32.5%����3����Ӧ�����ɵ���Һ��������������Ϊ37.6%��
�����Ʒ��Na2CO3������Ϊx����Na2CO3��Ӧ��H2SO4������Ϊy����Na2CO3��Ӧ���ɵ������Ƶ�����Ϊz��
Na2CO3+H2SO4�TNa2SO4+H2O+CO2��
106 98 142 44
x y z 2.2g
��1��
| 106 |
| 44 |
| x |
| 2.2g |
| 98 |
| 44 |
| y |
| 2.2g |
| 142 |
| 44 |
| z |
| 2.2g |
��Ʒ���������Ƶ�����Ϊ21.2g-5.3g=15.9g������NaOH��Ӧ��H2SO4������Ϊa����NaOH��Ӧ���ɵ������Ƶ�����Ϊb��
2NaOH+H2SO4�TNa2SO4+H2O
80 98 142
15.9g a b
| 80 |
| 98 |
| 15.9g |
| a |
| 80 |
| 142 |
| 15.9g |
| b |
��2��ϡ���������������
| 4.9g+19.5g |
| 75g |
��3����Ӧ�����ɵ���Һ��������������Ϊ
| 7.1g+28.2g |
| 21.2g+75g-2.2g |
�𣺣�1������Ʒ��Na2CO3������Ϊ5.3g����2��ϡ���������������32.5%����3����Ӧ�����ɵ���Һ��������������Ϊ37.6%��
�����������ѶȽϴ��ۺϿ�����ѧ����ͼ�������ʶ����������ȷͼ��ĺ��塢ע��ͼ��������ĵ�������ǽ����������ؼ���

��ϰ��ϵ�д�
�����Ŀ
ijУ�о���ѧϰС���ͬѧ����˼�ʵ�鷽������֤���������������������ɷֵĺ�����ʲô��ͬ���뽫ʵ�����������±��еĿո�
| ʵ�鲽�� | ʵ������ |
| 1�� ����ˮ���ռ���ƿ���������壬 ���ռ���ƿ������ | ���� |
| 2��������ʯ��ˮ�ֱ����ʢ�к����� ����Ϳ����ļ���ƿ�У��� | ������������ʯ��ˮ_________ |
| 3����ȼ�ŵ�ľ���Ž�������ƿ������ | ��Ϩ�����__________________ |
| 4����ͷ����Ƭ���� | �����ϳ���______________ |
���յó���ʵ�������__________________________________��
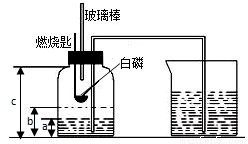
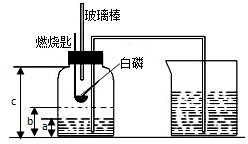 ijУ��ѧ�о���ѧϰС����ѧϰ�ˡ����������������ⶨ���Ļ����ϣ��Ľ��˽̲��е�ʵ�飬��Ƴ�����ͼ��ʾ��ʵ��װ�ã�ʵ�鲽�����£�
ijУ��ѧ�о���ѧϰС����ѧϰ�ˡ����������������ⶨ���Ļ����ϣ��Ľ��˽̲��е�ʵ�飬��Ƴ�����ͼ��ʾ��ʵ��װ�ã�ʵ�鲽�����£� ijУ�о���ѧϰС�鵽ʵ���ҽ���̽��ʵ�飮�������ü��ȸ�����صķ�����ȡ��������֤���������ʣ�
ijУ�о���ѧϰС�鵽ʵ���ҽ���̽��ʵ�飮�������ü��ȸ�����صķ�����ȡ��������֤���������ʣ�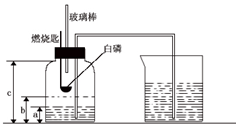 ijУ��ѧ�о���ѧϰС����ѧϰ�ˡ����������������ⶨ���Ļ����ϣ��Ľ��˽̲��е�ʵ�飬��Ƴ�����ͼ��ʾ��ʵ��װ�ã�ʵ�鲽�����£�
ijУ��ѧ�о���ѧϰС����ѧϰ�ˡ����������������ⶨ���Ļ����ϣ��Ľ��˽̲��е�ʵ�飬��Ƴ�����ͼ��ʾ��ʵ��װ�ã�ʵ�鲽�����£�