��Ŀ����
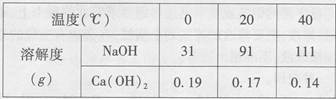
��2011��㶫��21�⣩�±���NaOH��Ca(OH)2���ܽ�����ݣ���ش��������⡣

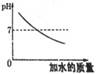
��1���ӱ������ݿ��Ի�õ���Ϣ��___________��дһ������
��2����80��ʱNaOH�ı�����Һ������20�棬���Կ�����������_______������20����Ca(OH)2�ı�����Һ������Һ���������м���һ����CaO��õ�����Һ������Һ������ʱ��Һ����������������______�ף��������������
��3��ij��ȤС��Բ��ֱ��ʵ��������ƹ�������ᴿ����������²������̣���ش�
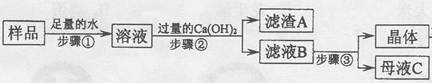

������ٷ�Ӧ�Ļ�ѧ����ʽΪ________���������Ca(OH)2��Ŀ����______________��
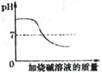
������ҺB�е�������__________��_________��д��ѧʽ����������������ľ�������Ǽ���Ũ����_______�����ˡ�


��1���ӱ������ݿ��Ի�õ���Ϣ��___________��дһ������
��2����80��ʱNaOH�ı�����Һ������20�棬���Կ�����������_______������20����Ca(OH)2�ı�����Һ������Һ���������м���һ����CaO��õ�����Һ������Һ������ʱ��Һ����������������______�ף��������������
��3��ij��ȤС��Բ��ֱ��ʵ��������ƹ�������ᴿ����������²������̣���ش�


������ٷ�Ӧ�Ļ�ѧ����ʽΪ________���������Ca(OH)2��Ŀ����______________��
������ҺB�е�������__________��_________��д��ѧʽ����������������ľ�������Ǽ���Ũ����_______�����ˡ�
��1���������Ƶ��ܽ�����¶ȵ����߶�����2����Һ���о�������������3��Na2CO3+10H2O==Na2CO3��10H2O����̼���Ƴ�ȥ��NaOH��Ca(OH)2������
��������1�����ñ��������������������Ƶ��ܽ����ֵ���ҵ���ص���Ϣ��
��2�������������Ƶ��ܽ�����¶Ƚ��Ͷ���С��֪ʶ�Լ����������ܽ�����¶����߶���С��ͬʱ�����ʯ������ˮ���ȵ�֪ʶ������⣮
��3��I�����ʵ��������Ƴ��Ậ��̼���ƣ�̼�������������Ʒ�Ӧ������̼������������ƣ��������������ƻ��̼��������ĸ��ɾ�һЩ��
II����ҺB�лẬ��ʣ����������Ƽ����������������ʣ����������ƺ��������Ƶ��ܽ�����¶ȵĹ�ϵ��ȷ���������ǵķ�ʽ��
��𣺽⣺��1����ͬ�¶��������������������Ƶ��ܽ�ȴ�С����ܴ�
�ʴ�Ϊ���������Ƶ��ܽ��ԶԶ�����������Ƶ��ܽ�ȣ�
��2�������������Ƶ��ܽ�����¶Ƚ��Ͷ���С���ʽ��»�ʹ��Һ�������ʣ���������Һˮ�������Һ�¶����ߣ����������ܽ�����¶����߶���С���ʿ��γɸ���ʱ�ı�����Һ������ҺҪ�ȵ���ʱ������������С��
�ʴ�Ϊ����Һ�����ǣ���
��3��I�����ʵ��������Ƴ��������������������̼��Ӧ����̼���ƣ�̼�������������Ʒ�Ӧ������̼������������ƣ�Ϊ��̼��������ĸ��ɾ�һЩ�������������������ƣ�
�ʴ�Ϊ��Na2CO3+Ca��OH��2=CaCO3��+2NaOH������Һ�е�̼������ȫת��Ϊ�������ƣ�
II��������Һ�м����˹������������ƣ�������Һ�лẬ�������������������ƣ���Ϊ���������ʵ��ܽ�����¶ȵı仯����ܴʿɲ��ý��½ᾧ�ķ�ʽ���з��룮
�ʴ�Ϊ��Ca��OH��2��NaOH�����½ᾧ
��2�������������Ƶ��ܽ�����¶Ƚ��Ͷ���С��֪ʶ�Լ����������ܽ�����¶����߶���С��ͬʱ�����ʯ������ˮ���ȵ�֪ʶ������⣮
��3��I�����ʵ��������Ƴ��Ậ��̼���ƣ�̼�������������Ʒ�Ӧ������̼������������ƣ��������������ƻ��̼��������ĸ��ɾ�һЩ��
II����ҺB�лẬ��ʣ����������Ƽ����������������ʣ����������ƺ��������Ƶ��ܽ�����¶ȵĹ�ϵ��ȷ���������ǵķ�ʽ��
��𣺽⣺��1����ͬ�¶��������������������Ƶ��ܽ�ȴ�С����ܴ�
�ʴ�Ϊ���������Ƶ��ܽ��ԶԶ�����������Ƶ��ܽ�ȣ�
��2�������������Ƶ��ܽ�����¶Ƚ��Ͷ���С���ʽ��»�ʹ��Һ�������ʣ���������Һˮ�������Һ�¶����ߣ����������ܽ�����¶����߶���С���ʿ��γɸ���ʱ�ı�����Һ������ҺҪ�ȵ���ʱ������������С��
�ʴ�Ϊ����Һ�����ǣ���
��3��I�����ʵ��������Ƴ��������������������̼��Ӧ����̼���ƣ�̼�������������Ʒ�Ӧ������̼������������ƣ�Ϊ��̼��������ĸ��ɾ�һЩ�������������������ƣ�
�ʴ�Ϊ��Na2CO3+Ca��OH��2=CaCO3��+2NaOH������Һ�е�̼������ȫת��Ϊ�������ƣ�
II��������Һ�м����˹������������ƣ�������Һ�лẬ�������������������ƣ���Ϊ���������ʵ��ܽ�����¶ȵı仯����ܴʿɲ��ý��½ᾧ�ķ�ʽ���з��룮
�ʴ�Ϊ��Ca��OH��2��NaOH�����½ᾧ

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ