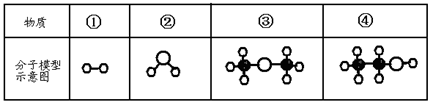
ΧβΡΩΡΎ»ί
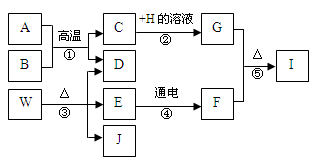
AΓΪJΖ÷±πΈΣΨ≈ΡξΦΕΜ·―ß―ßΙΐΒΡ≤ΜΆ§¥ΩΨΜΈοΘ§ΥϋΟ«¥φ‘Ύ”“œ¬ΆΦΥυ ΨΒΡΉΣΜ·ΙΊœΒΓΘ“―÷ΣAΈΣΧζ–βΒΡ÷ς“Σ≥…Ζ÷Θ§HΒΡ»ή“Κ≥ άΕ…ΪΘ§IΈΣΚΎ…ΪΙΧΧεΘ§HΒΡœύΕ‘Ζ÷Ή”÷ ΝΩ «IΒΡΝΫ±ΕΓΘ≥ΘΈ¬œ¬BΓΔDΓΔFΓΔJΨυΈΣΈό…ΪΤχΧεΘ§Τδ÷–J «“Μ÷÷”–¥ΧΦΛ–‘ΤχΈΕΒΡΤχΧεΘ§ΤδΥ°»ή“Κœ‘Φν–‘ΓΘΘ®Ζ¥”ΠΔΎΓΔΔήΒΡΗω±π…ζ≥…Έο“―¬‘»ΞΘ©
Θ®1Θ©–¥≥ωΜ·―ß ΫΘΚ
E________Θ§ I ________Θ§
J ΓΘ
Θ®2Θ©WΉι≥…÷–Κ§”–―τάκΉ” «Θ®ΧνΖϊΚ≈Θ©_________ ΓΘ
Θ®3Θ©–¥≥ωΖ¥”ΠΔΌΚΆΔΎΒΡΜ·―ßΖΫ≥Χ ΫΘΚ
ΔΌ_______________________________________________
ΔΎ

Θ®1Θ©–¥≥ωΜ·―ß ΫΘΚ
E________Θ§ I ________Θ§
J ΓΘ
Θ®2Θ©WΉι≥…÷–Κ§”–―τάκΉ” «Θ®ΧνΖϊΚ≈Θ©_________ ΓΘ
Θ®3Θ©–¥≥ωΖ¥”ΠΔΌΚΆΔΎΒΡΜ·―ßΖΫ≥Χ ΫΘΚ
ΔΌ_______________________________________________
ΔΎ
–¥≥ωΜ·―ß ΫΘΚ
E__H2O______Θ§ I _CuO_______Θ§
J NH3 ΓΘ
Θ®2Θ©WΉι≥…÷–Κ§”–―τάκΉ” «Θ®ΧνΖϊΚ≈___NH4+______ ΓΘ
Θ®3Θ©–¥≥ωΖ¥”ΠΔΌΚΆΔΎΒΡΜ·―ßΖΫ≥Χ ΫΘΚ
ΔΌ____ Fe2O3+3CO 2Fe+3CO2____
2Fe+3CO2____
ΔΎ Fe+CuSO4=Cu+FeSO4
E__H2O______Θ§ I _CuO_______Θ§
J NH3 ΓΘ
Θ®2Θ©WΉι≥…÷–Κ§”–―τάκΉ” «Θ®ΧνΖϊΚ≈___NH4+______ ΓΘ
Θ®3Θ©–¥≥ωΖ¥”ΠΔΌΚΆΔΎΒΡΜ·―ßΖΫ≥Χ ΫΘΚ
ΔΌ____ Fe2O3+3CO
 2Fe+3CO2____
2Fe+3CO2____ΔΎ Fe+CuSO4=Cu+FeSO4
ΘΚΘ®1Θ©E‘ΎΆ®ΒγΧθΦΰœ¬…ζ≥…ΤχΧεFΘ§Ω…ΆΤΕœEΈΣΥ°H2OΘΜJ «“Μ÷÷ΨΏ”–¥ΧΦΛ–‘ΤχΈΕΒΡΤχΧεΘ§Ρή Ι Σ»σΒΡΚλ…Ϊ ·»ο ‘÷Ϋ±δάΕΘ§Ω…»ΖΕ®JΈΣNH3ΘΜAΈΣΧζ–βΒΡ÷ς“Σ≥…Ζ÷Θ§‘ρA «Fe2O3Θ§”…B «Έό…ΪΤχΧεΘ§ΫαΚœΉΣΜ·ΙΊœΒΩρΆΦ‘ΎΗΏΈ¬ΧθΦΰœ¬Ρή”κFe2O3ΖΔ…ζΖ¥”ΠΘ§ΨΏ”–ΜΙ‘≠–‘Θ§…ζ≥…D“≤ «Έό…ΪΤχΧεΘ§‘ρBΈΣCOΘ§DΈΣCO2Θ§CΈΣFeΘΜHΈΣάΕ…Ϊ»ή“ΚΘ§‘ρHΈΣCuSO4Θ§”κΧζΖ¥”Π…ζ≥…ΒΡGΈΣCuΘ§EΆ®Βγ…ζ≥…ΒΡFΡή”κΆ≠Ζ¥”ΠΘ§‘ρFΈΣO2Θ§IΈΣCuOΘΜ
Θ®2Θ©”…Θ®1Θ©“―÷ΣDΈΣCO2Θ§EΈΣΥ°H2OΘ§JΈΣΑ±ΤχNH3Θ§ΗυΨίΉΣΜ·ΙΊœΒΘ§WΦ”»»…ζ≥…CO2ΓΔH2OΓΔNH3Θ§Ι WΉι≥…÷–Κ§”–ΒΡ―τάκΉ” «NH4+ΘΜ
Θ®3Θ©AΈΣFe2O3Θ§BΈΣCOΘ§Ζ¥”ΠΔΌΈΣCOΜΙ‘≠Fe2O3ΒΡΖ¥”ΠΘ§Μ·―ßΖΫ≥Χ ΫΈΣΘΚFe2O3+3CO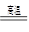 2Fe+3CO2ΘΜ
2Fe+3CO2ΘΜ
CΈΣΧζΓΔ»ή“ΚHΈΣΩ…»ή–‘Ά≠―ΈΒΡάΕ…Ϊ»ή“ΚΘ§Ήν≥ΘΦϊΒΡΈΣΝρΥαΆ≠»ή“ΚΘΜ“ρ¥ΥΖ¥”ΠΔΎΈΣΧζ÷ΟΜΜΝρΥαΆ≠÷–ΒΡΆ≠…ζ≥…ΝρΥα―«ΧζΘΜ
Μ·―ßΖΫ≥Χ ΫΈΣΘΚFe+CuSO4=Cu+FeSO4ΘΜ
Ι ¥πΑΗΈΣΘΚΘ®1Θ©H2OΘ§CuOΘ§NH3ΘΜ
Θ®2Θ©NH4+ΘΜ
Θ®3Θ©ΔΌFe2O3+3CO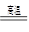 2Fe+3CO2Θ§ΔΎFe+CuSO4=Cu+FeSO4Θ°
2Fe+3CO2Θ§ΔΎFe+CuSO4=Cu+FeSO4Θ°
Θ®2Θ©”…Θ®1Θ©“―÷ΣDΈΣCO2Θ§EΈΣΥ°H2OΘ§JΈΣΑ±ΤχNH3Θ§ΗυΨίΉΣΜ·ΙΊœΒΘ§WΦ”»»…ζ≥…CO2ΓΔH2OΓΔNH3Θ§Ι WΉι≥…÷–Κ§”–ΒΡ―τάκΉ” «NH4+ΘΜ
Θ®3Θ©AΈΣFe2O3Θ§BΈΣCOΘ§Ζ¥”ΠΔΌΈΣCOΜΙ‘≠Fe2O3ΒΡΖ¥”ΠΘ§Μ·―ßΖΫ≥Χ ΫΈΣΘΚFe2O3+3CO
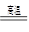 2Fe+3CO2ΘΜ
2Fe+3CO2ΘΜCΈΣΧζΓΔ»ή“ΚHΈΣΩ…»ή–‘Ά≠―ΈΒΡάΕ…Ϊ»ή“ΚΘ§Ήν≥ΘΦϊΒΡΈΣΝρΥαΆ≠»ή“ΚΘΜ“ρ¥ΥΖ¥”ΠΔΎΈΣΧζ÷ΟΜΜΝρΥαΆ≠÷–ΒΡΆ≠…ζ≥…ΝρΥα―«ΧζΘΜ
Μ·―ßΖΫ≥Χ ΫΈΣΘΚFe+CuSO4=Cu+FeSO4ΘΜ
Ι ¥πΑΗΈΣΘΚΘ®1Θ©H2OΘ§CuOΘ§NH3ΘΜ
Θ®2Θ©NH4+ΘΜ
Θ®3Θ©ΔΌFe2O3+3CO
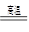 2Fe+3CO2Θ§ΔΎFe+CuSO4=Cu+FeSO4Θ°
2Fe+3CO2Θ§ΔΎFe+CuSO4=Cu+FeSO4Θ°
ΝΖœΑ≤αœΒΝ–¥πΑΗ
œύΙΊΧβΡΩ
 ¥ζ±μ«β‘≠Ή”Θ§
¥ζ±μ«β‘≠Ή”Θ§ ¥ζ±μ―θ‘≠Ή”Θ§
¥ζ±μ―θ‘≠Ή”Θ§ ¥ζ±μΧΦ‘≠Ή”Θ§‘ρœ¬Ν–”–ΙΊΥΒΖ®’ΐ»ΖΒΡ «ΘΚ
¥ζ±μΧΦ‘≠Ή”Θ§‘ρœ¬Ν–”–ΙΊΥΒΖ®’ΐ»ΖΒΡ «ΘΚ