题目内容
某同学为探究石灰石样品中碳酸钙的质量分数(杂质不与酸反应),向6.0g石灰石样品中逐滴加入稀盐酸至不再产生气泡为止,共生成二氧化碳气体1.11L(该条件下二氧化碳气体的密度为1.977g/L).试计算:(1)该反应生成二氧化碳的质量为多少?(精确至0.1g)
(2)求该石灰石中碳酸钙的质量分数为多少?(精确到0.1%)
【答案】分析:(1)根据物理公式质量=密度×体积计算即可;
(2)根据碳酸钙和盐酸反应的化学方程式,得出各物质之间的质量比,列出比例式,即可计算出石灰石样品中碳酸钙的质量,然后根据质量分数公式计算即可;
解答:解:(1)反应生成二氧化碳的质量为:1.11L×1.977g/L≈2.2g.
(2)设石灰石中碳酸钙的质量为x.
CaCO3+2HCl=CaCl2+H2O+CO2↑
100 44
x 2.2g
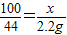
解之得:x=5g,
该石灰石样品中碳酸钙的质量分数为: ×100%≈83.3%.
×100%≈83.3%.
答:(1)该反应生成二氧化碳的质量为2.2g;
(2)该石灰石样品中碳酸钙的质量分数为83.3%.
点评:本题主要考查根据化学方程式的计算,难度较小.石灰石为混合物,石灰石中除了碳酸钙之外还有杂质,不能将石灰石的质量直接代入化学方程式进行计算.
(2)根据碳酸钙和盐酸反应的化学方程式,得出各物质之间的质量比,列出比例式,即可计算出石灰石样品中碳酸钙的质量,然后根据质量分数公式计算即可;
解答:解:(1)反应生成二氧化碳的质量为:1.11L×1.977g/L≈2.2g.
(2)设石灰石中碳酸钙的质量为x.
CaCO3+2HCl=CaCl2+H2O+CO2↑
100 44
x 2.2g
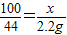
解之得:x=5g,
该石灰石样品中碳酸钙的质量分数为:
 ×100%≈83.3%.
×100%≈83.3%.答:(1)该反应生成二氧化碳的质量为2.2g;
(2)该石灰石样品中碳酸钙的质量分数为83.3%.
点评:本题主要考查根据化学方程式的计算,难度较小.石灰石为混合物,石灰石中除了碳酸钙之外还有杂质,不能将石灰石的质量直接代入化学方程式进行计算.

练习册系列答案
相关题目