��Ŀ����
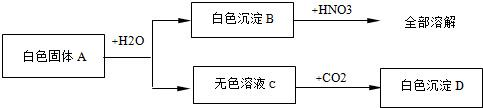
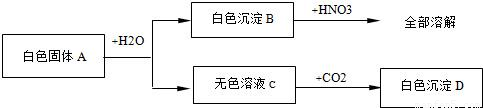
(4��).��һ����ɫ��ĩ�����ܺ���CuSO4��NaCl��AgCl��Na2SO4��Na2CO3�е�һ�ֻ��֣��ֽ������µ�ʵ�飺��1������ɫ��ĩ����������ˮ�У��õ���ɫ������Һ����2������ɫ������Һ�м����Ȼ�����Һ���а�ɫ�������ɡ���3�����ɫ�����м���������ϡ���ᣬ�в��ֳ����ܽ⣬��������ʹ����ʯ��ˮ����ǵ����塣����������ʵ���ð�ɫ��ĩ��һ������________�����ܺ���_______��һ��û��_________�����ݣ�1���й�����ȫ�ܽ⣬��AgCl������ˮ����ԭ��ĩ��һ��û��AgCl��ͬʱ�õ���ɫ��Һ����CuSO4��Һ����ɫ����ԭ��ĩ��һ��û��CuSO4���ٸ��ݣ�2���м��Ȼ���������ɫ����������Һ�в����ڱ����ӣ���ԭ��ĩ����Na2SO4����Na2CO3���ٸ��ݣ�3����ɫ�������в��ֳ����ܽⲢ�ų����壬�����Ϊ̼�ᱵ��������ԭ��ĩ��һ����Na2CO3�������ᱵ�������ᣬ��ԭ��ĩ��һ����Na2SO4��

��7�֣���һ����ɫ�Ĺ����ĩ��������NaHCO3��NaOH�е�һ�ֻ���֣���ѧС�������ɽ�����ͼ��ʾ��ʵ�飺
���ϣ�1.�ƾ��Ƽ����¶�Լ500��600 �棻
2. NaOH 318.4���ۻ����ֽ⣬1390 ����ڲ��ֽ⣻
3. Na2CO3+CaCl2��CaCO3��+ 2NaCl
4. Na2CO3��Һ�ʼ��ԣ�CaCl2��Һ������
��1�����ʵ�鱨������ݣ�
| ʵ����� | ʵ������ | ʵ����� |
| ��ȡһ�����İ�ɫ��ĩ���ȣ�������������ͨ��С�ձ��У������������ʱ��ֹͣ���� ��С�ձ���ʢ�г����ʯ��ˮ�� | �Թ��ڱ�����ɫҺ�Σ�ʯ��ˮ����ǣ��Թ���ʣ���ɫ�������� | ��ĩ��һ������ ��Ӧ�Ļ�ѧ����ʽ�� |
| ��ȡʵ�����ʣ���ɫ�����������Թ��У����Թ��м���ϡ���� | | ʵ�����ʣ���ɫ������һ������ �� ��Ӧ�Ļ�ѧ����ʽ�� |
��7�֣���һ����ɫ�Ĺ����ĩ��������NaHCO3��NaOH�е�һ�ֻ���֣���ѧС�������ɽ�����ͼ��ʾ��ʵ�飺
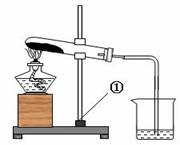
���ϣ�1.�ƾ��Ƽ����¶�Լ500��600 �棻
2. NaOH 318.4���ۻ����ֽ⣬1390 ����ڲ��ֽ⣻
3. Na2CO3+CaCl2��CaCO3��+ 2NaCl
4. Na2CO3��Һ�ʼ��ԣ�CaCl2��Һ������
��1�����ʵ�鱨������ݣ�
|
ʵ����� |
ʵ������ |
ʵ����� |
|
��ȡһ�����İ�ɫ��ĩ���ȣ�������������ͨ��С�ձ��У������������ʱ��ֹͣ���� ��С�ձ���ʢ�г����ʯ��ˮ�� |
�Թ��ڱ�����ɫҺ�Σ�ʯ��ˮ����ǣ��Թ���ʣ���ɫ��������
|
��ĩ��һ������
��Ӧ�Ļ�ѧ����ʽ��
|
|
��ȡʵ�����ʣ���ɫ�����������Թ��У����Թ��м���ϡ����
|
|
ʵ�����ʣ���ɫ������һ������ �� ��Ӧ�Ļ�ѧ����ʽ��
|
��2��Ϊȷ����ɫ��ĩ���Ƿ���NaOH�ķ����ǣ�ȡʵ�����ʣ���ɫ���������� ��