��Ŀ����
��2009?��ɽ��һģ��ij��ȤС��Ϊ���о�NaCl��KNO3���ܽ�ͽᾧ����������ʵ�飨�����£�����������£�
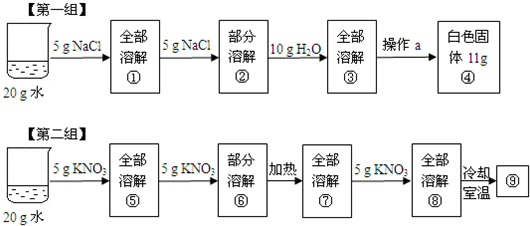
��1���������ʵ���������Ϊ
��2����һ��ʵ���в���aΪ
A���ܽ�NaCl����ʱ��û���ò���������
B������5g NaCl�������ֽ�������й���
C������ʱ��δ��ʱֹͣ���ȣ��й��彦��
D������������NaCl �������Ժ���ˮ��
��3������ʵ�������һ�����ڱ�����Һ���ǣ���������ţ���ͬ��

��1���������ʵ���������Ϊ
20%
20%
������������������
�������
����2����һ��ʵ���в���aΪ
����
����
�������Ȼ��ƹ������������ԭ������ǣ�����ĸ��ţ�D
D
��A���ܽ�NaCl����ʱ��û���ò���������
B������5g NaCl�������ֽ�������й���
C������ʱ��δ��ʱֹͣ���ȣ��й��彦��
D������������NaCl �������Ժ���ˮ��
��3������ʵ�������һ�����ڱ�����Һ���ǣ���������ţ���ͬ��
�ڢޢ�
�ڢޢ�
�����ʵ���������һ����ȵ��������
�����
��д��������һ�鼴�ɣ����������������е�֪ʶ���з������������ʵ���������Һ����������������ʵ�����������������Һָ��һ���¶�����һ�������ܼ��ﲻ�����ܽ�ij�����ʵ���Һ��
����⣺��1���������ʵ�����Ϊ5g���ܼ�������Ϊ20g���������ʵ���������Ϊ��
��100%=20%���ڶ������������γ�������صı�����Һ�������¶�����ؼ����ܽ⣬�ʽ����¶�ʱ����������صľ��壬���20%���о���������
��2��Ҫ�õ��Ȼ��Ƶľ��壬���Բ��������ܼ��ķ�����A�ܽ�NaCl����ʱ��û���ò��������裬��Ӱ���ܽ�����������B����ʱ��ֽ���в����Ĺ��壬��ʹ�Ȼ��Ƶ��������٣�C����ʱ��δ��ʱֹͣ���ȣ��й��彦������ʹ�Ȼ��Ƶ��������٣�D����������NaCl �������Ժ���ˮ�֣���ʹ�Ȼ��Ƶ�����ƫ���������D��
��3���õ�����Һ�ڲ����ܽ��Ȼ��ƣ��DZ�����Һ���õ�����Һ�����ܽ�����أ��DZ�����Һ��������ȴ��������ص���Һ���DZ�����Һ���٢ݶ�����20gˮ���ܽ�5g���ʣ���������������һ����ͬ������ڢޢᣬ����ݣ�
| 5g |
| 25g |
��2��Ҫ�õ��Ȼ��Ƶľ��壬���Բ��������ܼ��ķ�����A�ܽ�NaCl����ʱ��û���ò��������裬��Ӱ���ܽ�����������B����ʱ��ֽ���в����Ĺ��壬��ʹ�Ȼ��Ƶ��������٣�C����ʱ��δ��ʱֹͣ���ȣ��й��彦������ʹ�Ȼ��Ƶ��������٣�D����������NaCl �������Ժ���ˮ�֣���ʹ�Ȼ��Ƶ�����ƫ���������D��
��3���õ�����Һ�ڲ����ܽ��Ȼ��ƣ��DZ�����Һ���õ�����Һ�����ܽ�����أ��DZ�����Һ��������ȴ��������ص���Һ���DZ�����Һ���٢ݶ�����20gˮ���ܽ�5g���ʣ���������������һ����ͬ������ڢޢᣬ����ݣ�
���������⿼���˱�����Һ�벻������Һ���жϣ���ɴ��⣬�����������е�֪ʶ���У�

��ϰ��ϵ�д�
�����Ŀ
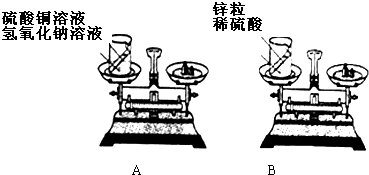 ��2009?��ɽ��һģ��Ϊ�о����ʷ�����ѧ�仯��ǰ���������Ƿ����ı䣬С����С����²������̽����
��2009?��ɽ��һģ��Ϊ�о����ʷ�����ѧ�仯��ǰ���������Ƿ����ı䣬С����С����²������̽����