��Ŀ����
��1���뽫���ж����мӵ������ת��Ϊǡ���Ļ�ѧ������ں���ĺ����ϣ������DZ�ʯ��һ�֣�������Ҫ�ɷ���������������������Ԫ�صĻ��ϼ�Ϊ+3�� ���������������� �� ��Ӳ�Ƚ����ڽ��ʯ ��һЩ�������ڻ��������������� �����ʶ�����ɫ���׳�����ʯ��
��2����������ˮƽ����ߣ������е�˽�ҳ�Խ��Խ�ࡣ
������������̥�������� ��
A���л��ϳɲ��� B���������� C�����ϲ��� D��������
��������·���ߴ����ͭ�Ƶģ��������ý���ͭ����չ�Ժ� ��
�۽����3.15������������ҵ�����˲�С�����ܶ�����ʹ����������Ƭ�����³�������ζ���������ɲ�ͬ��Է���������̼�⻯���P��ǽ�����������ɵģ��ɴ��ƶ��������� �����������������
���������м����������Ҵ��͵õ��Ҵ����ͣ�ʹ���Ҵ����͵��ŵ� ����һ�㣩��
�ݳ���Ǧ���س��ķ�Ӧ��������ʡ�ԣ���2PbSO4+2H2O =Pb+2H2SO4 + ��
��1��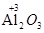
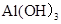 C
C 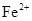
��2����A �ڵ����� �� �����
�ܼ�����Ⱦ����Լʯ����Դ�ȣ� ��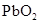
��������
�����������1����������������Ԫ�صĻ��ϼ�Ϊ�����۱�ʾ����Ϊ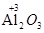 ����������Ϊ
����������Ϊ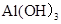 �����ʯ�Ļ�ѧ���Ϊ̼����������Ϊ
�����ʯ�Ļ�ѧ���Ϊ̼����������Ϊ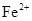 ����2�����������л��ϳɲ��ϣ���ͭ�����������������������õĵ����Ժ���չ�ԣ��������֪�����ɶ����л�����ɣ����ڻ������Ҵ����Ϳ�������ȼ��Ч�ʣ�������Ⱦ����Լ��Դ���ݸ��������غ㶨�ɣ���ѧ��Ӧǰ�������ԭ�������������仯�����Ը�����Ϊ
����2�����������л��ϳɲ��ϣ���ͭ�����������������������õĵ����Ժ���չ�ԣ��������֪�����ɶ����л�����ɣ����ڻ������Ҵ����Ϳ�������ȼ��Ч�ʣ�������Ⱦ����Լ��Դ���ݸ��������غ㶨�ɣ���ѧ��Ӧǰ�������ԭ�������������仯�����Ը�����Ϊ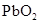 ��
��
���㣺������ѧ����
�����������Ļ�ѧ��������漰�Ļ�ѧ��ʶ�����п����ȵ㣬������ѡ����ʹ����г��֣�ע����ϸ���⡣

��1���뽫���ж����мӵ������ת��Ϊǡ���Ļ�ѧ������ں���ĺ����ϣ������DZ�ʯ��һ�֣�������Ҫ�ɷ���������������������Ԫ�صĻ��ϼ�Ϊ+3�� ���������������� �� ��Ӳ�Ƚ����ڽ��ʯ ��һЩ�������ڻ��������������� �����ʶ�����ɫ���׳�����ʯ��
��2����������ˮƽ����ߣ������е�˽�ҳ�Խ��Խ�ࡣ
������������̥�������� ��
| A���л��ϳɲ��� | B���������� | C�����ϲ��� | D�������� |
�۽����3.15������������ҵ�����˲�С�����ܶ�����ʹ����������Ƭ�����³�������ζ���������ɲ�ͬ��Է���������̼�⻯���P��ǽ�����������ɵģ��ɴ��ƶ��������� �����������������
���������м����������Ҵ��͵õ��Ҵ����ͣ�ʹ���Ҵ����͵��ŵ� ����һ�㣩��
�ݳ���Ǧ���س��ķ�Ӧ��������ʡ�ԣ���2PbSO4+2H2O =Pb+2H2SO4 + ��
 14�������д����л�ѧ�������û�ѧ֪ʶ�ش����������е����⣺
14�������д����л�ѧ�������û�ѧ֪ʶ�ش����������е����⣺