��Ŀ����
��2008?տ����������ҵ�ǹ��ҹ�ҵ�Ļ�����2007���ҹ��ֲָ����ӽ�5�ڶ֣�������ǰ�У�տ���Ķ�����Ҳ������ǧ��ָ��������⽫��տ���ľ��ô����ʵķ�Ծ��ijУ��ȤС��ȡij�ָ����������飬����㣺��1��ȡ����������ֻ�������ʺ�̼���ʣ���ĩ100g���������г��ȼ�գ��õ�CO2����4.4g���˸�����ĩ��̼����������Ϊ______��������0.1%����
��2���ֱ����ķݲ�ͬ�����ĸ�����ĩ�м���100gϡ������Һ����ַ�Ӧ��õ�ʵ���������±���ʾ��
���� ���� | ʵ��1 | ʵ��2 | ʵ��3 | ʵ��4 |
| ������ĩ���� | 2.84g | 4.25g | 6.23g | 7.51g |
| ����H2������ | 0.10g | 0.15g | 0.20g | 0.20g |
������ʵ��3�з�Ӧ�����õ���Һ�����ʵ�����������
���𰸡���������1��������������ȼ��ʱ�����е�̼��������Ӧ���ɶ�����̼���ɸ������ɵĶ�����̼���������̼���������������Ʒ��̼������������
��2���ȷ����������ݣ���ȷ������������ȫ��Ӧ���ٸ�����֪����������������ʵ����������ɵ������������������ٽ�һ�������Ӧ����Һ�����ʵ�����������
�����1���⣺��100g������̼������ΪX
C+O2 CO2
CO2
12 44
X 4.4g
12×4.4g=44×X��
��ã�X=1.2g
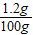 ×100%=1.2%
×100%=1.2%
��2���⣺ͨ�������Ƚ�ʵ��3��ʵ��4����֪��ʵ��3ʱ����������ȫ��Ӧ��������ȫΪ����������
��ʵ��3����������������ΪX��������������ΪY���������������ʵ�����ΪZ��
Fe+H2SO4�TFeSO4+H2��
56 98 152 2
Y Z x 0.2g
��0.2g×98=2×Z ��ã�Z=9.8g
0.2g×152=2×X ��ã�X=15.2g
0.2g×56=2×y ��ã�y=5.6g
��Һ��������������Ϊ��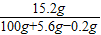 ×100%=14.4%
×100%=14.4%
��������Һ�����ʵ�����Ϊ9.8g��ʵ��3�з�Ӧ�����õ���Һ�����ʵ���������14.4%��
�ʴ�Ϊ����1��1.2%����2����9.8g����14.4%��
�������Ա����е����ݽ��з������ҳ�Ӧ�����ڼ�������ݣ��ǽ���֮�ؼ����ڣ�Ҳ��Ҫ�ص�ѵ�����������ݣ�
��2���ȷ����������ݣ���ȷ������������ȫ��Ӧ���ٸ�����֪����������������ʵ����������ɵ������������������ٽ�һ�������Ӧ����Һ�����ʵ�����������
�����1���⣺��100g������̼������ΪX
C+O2
 CO2
CO212 44
X 4.4g
12×4.4g=44×X��
��ã�X=1.2g
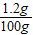 ×100%=1.2%
×100%=1.2%��2���⣺ͨ�������Ƚ�ʵ��3��ʵ��4����֪��ʵ��3ʱ����������ȫ��Ӧ��������ȫΪ����������
��ʵ��3����������������ΪX��������������ΪY���������������ʵ�����ΪZ��
Fe+H2SO4�TFeSO4+H2��
56 98 152 2
Y Z x 0.2g
��0.2g×98=2×Z ��ã�Z=9.8g
0.2g×152=2×X ��ã�X=15.2g
0.2g×56=2×y ��ã�y=5.6g
��Һ��������������Ϊ��
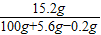 ×100%=14.4%
×100%=14.4%��������Һ�����ʵ�����Ϊ9.8g��ʵ��3�з�Ӧ�����õ���Һ�����ʵ���������14.4%��
�ʴ�Ϊ����1��1.2%����2����9.8g����14.4%��
�������Ա����е����ݽ��з������ҳ�Ӧ�����ڼ�������ݣ��ǽ���֮�ؼ����ڣ�Ҳ��Ҫ�ص�ѵ�����������ݣ�

��ϰ��ϵ�д�
 �żӾ���ϵ�д�
�żӾ���ϵ�д�
�����Ŀ
��2008?տ����������ҵ�ǹ��ҹ�ҵ�Ļ�����2007���ҹ��ֲָ����ӽ�5�ڶ֣�������ǰ�У�տ���Ķ�����Ҳ������ǧ��ָ��������⽫��տ���ľ��ô����ʵķ�Ծ��ijУ��ȤС��ȡij�ָ����������飬����㣺
��1��ȡ����������ֻ�������ʺ�̼���ʣ���ĩ100g���������г��ȼ�գ��õ�CO2����4.4g���˸�����ĩ��̼����������Ϊ______��������0.1%����
��2���ֱ����ķݲ�ͬ�����ĸ�����ĩ�м���100gϡ������Һ����ַ�Ӧ��õ�ʵ���������±���ʾ��
�ټ���������Һ�����ʵ�����______g
������ʵ��3�з�Ӧ�����õ���Һ�����ʵ�����������
��1��ȡ����������ֻ�������ʺ�̼���ʣ���ĩ100g���������г��ȼ�գ��õ�CO2����4.4g���˸�����ĩ��̼����������Ϊ______��������0.1%����
��2���ֱ����ķݲ�ͬ�����ĸ�����ĩ�м���100gϡ������Һ����ַ�Ӧ��õ�ʵ���������±���ʾ��
���� ���� | ʵ��1 | ʵ��2 | ʵ��3 | ʵ��4 |
| ������ĩ���� | 2.84g | 4.25g | 6.23g | 7.51g |
| ����H2������ | 0.10g | 0.15g | 0.20g | 0.20g |
������ʵ��3�з�Ӧ�����õ���Һ�����ʵ�����������
��2008?տ����������ҵ�ǹ��ҹ�ҵ�Ļ�����2007���ҹ��ֲָ����ӽ�5�ڶ֣�������ǰ�У�տ���Ķ�����Ҳ������ǧ��ָ��������⽫��տ���ľ��ô����ʵķ�Ծ��ijУ��ȤС��ȡij�ָ����������飬����㣺
��1��ȡ����������ֻ�������ʺ�̼���ʣ���ĩ100g���������г��ȼ�գ��õ�CO2����4.4g���˸�����ĩ��̼����������Ϊ______��������0.1%����
��2���ֱ����ķݲ�ͬ�����ĸ�����ĩ�м���100gϡ������Һ����ַ�Ӧ��õ�ʵ���������±���ʾ��
�ټ���������Һ�����ʵ�����______g
������ʵ��3�з�Ӧ�����õ���Һ�����ʵ�����������
��1��ȡ����������ֻ�������ʺ�̼���ʣ���ĩ100g���������г��ȼ�գ��õ�CO2����4.4g���˸�����ĩ��̼����������Ϊ______��������0.1%����
��2���ֱ����ķݲ�ͬ�����ĸ�����ĩ�м���100gϡ������Һ����ַ�Ӧ��õ�ʵ���������±���ʾ��
���� ���� | ʵ��1 | ʵ��2 | ʵ��3 | ʵ��4 |
| ������ĩ���� | 2.84g | 4.25g | 6.23g | 7.51g |
| ����H2������ | 0.10g | 0.15g | 0.20g | 0.20g |
������ʵ��3�з�Ӧ�����õ���Һ�����ʵ�����������