��Ŀ����
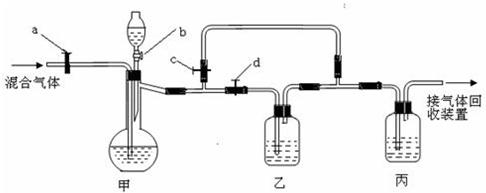
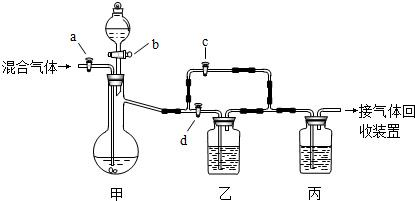
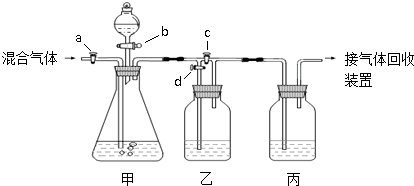
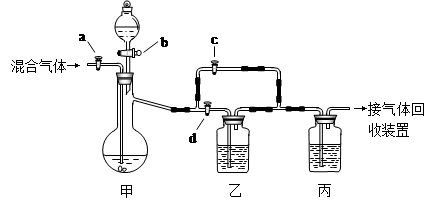
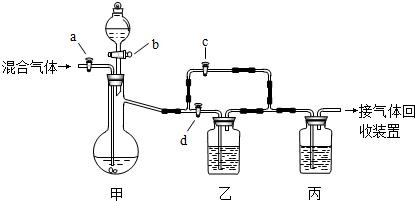
�������װ�ý�һ������CO2��CO�Ļ��������з�����ͼ�е�a��b��c��d��Ϊ���������Կ��������ͨ����Һ��ļ��룬ʵ��ǰ�������ѹرա���ѡ�����˵��Լ��������ʵ�顣
��ѡ�õ��Լ��У���ϡ�����Ũ���������������Һ�ܳ����ʯ��ˮ���Լ���������

ʵ��������������У�
��1���ر�b��c����a��d������з�����Ӧ�Ļ�ѧ����ʽΪ_______________��������
��Ϊ (���������)������װ���е������� ______ ��˵����װ����
��Ӧ�dz�ֵġ� �˲�ʵ����ռ�����������  ��
��
��2���ڼ�װ�õķ�Һ© ���м����Լ��٣�Ȼ��_________________________�������ռ���
���м����Լ��٣�Ȼ��_________________________�������ռ���
��һ�����塣
��1��CO2 + 2NaOH= Na2CO3 + H2O �� ʯ��ˮ������� һ����̼����CO��
��2�� �ر�a��d����b��c���÷�Һ©������Һ�������������ٲ�������ʱ��
�ر�b

��ϰ��ϵ�д�
 ���������ν�ϵ�д�
���������ν�ϵ�д�
�����Ŀ