��Ŀ����
����Ŀ��ʵ���ҳ��õ�������ȡװ�����¡���ش��������:
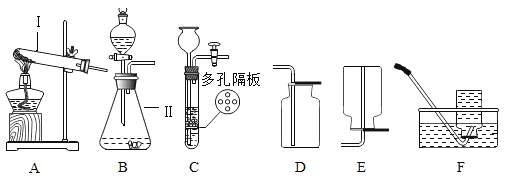
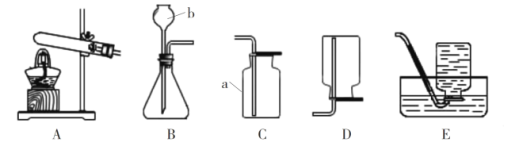
(1)д���������������: I_______________����װ��A��ҩƷ���������ȡ������������һ��Ʒ________��Cװ����ȡ������ŵ�Ϊ________________��
(2)ʵ���Ҽȿ�����ȡCO2���ֿ�����ȡO2����Ϊ�����ķ���װ��Ϊ_______________(��װ�ñ��)��ʵ������ȡCO2�Ļ�ѧ����ʽΪ_____________________________��
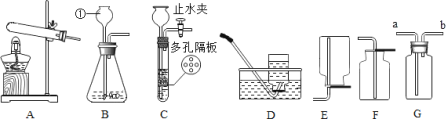
(3)ijͬѧ���ÿ�������Һ���ռ�������̼(����ͼ)������ʱ����ȼ�ŵ�ľ�����ڲ�����___________(�a����b��)�ˣ����Ϩ�������ˡ�

(4)��һ������������̼���Թܵ��������1-2�η�̪�ij���ʯ��ˮ���ձ���(����ͼ��ʾ)��ʵ���пɹ۲쵽��������________________��
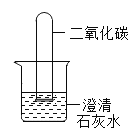
���𰸡��Թ� һ���� ���Կ��Ʒ�Ӧ�ķ�����ֹͣ B CaCO3+2HCl=CaCl2+H2O+CO2�� b ����ʯ��ˮ����ǣ��Թ���Һ������
��������
��1������I���Թܣ���װ��A��ҩƷ���������ȡ�������Թܿ���Ҫ��һ��������ֹ����ʱ������ط�ĩ���뵼�ܣ�Cװ����ȡ������ŵ�Ϊ�����Կ��Ʒ�Ӧ�ķ�����ֹͣ������Թܣ�һ���������Կ��Ʒ�Ӧ�ķ�����ֹͣ��
��2��ʵ���ҳ��ô���ʯ����ʯ��ʯ����ϡ���ᷴӦ��ȡ������̼���ù���������ȡ�����������ڹ̡�Һ�����ͣ�������װ��B������װ�ã�̼��ƺ�ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2�������B��CaCO3+2HCl=CaCl2+H2O+CO2����
��3����Ϊ������̼���ܶȱȿ�����ȼ��Ҳ��֧��ȼ�գ�ijͬѧ���ÿ�������Һ���ռ�������̼����ͼG����Ӧ��a��ͨ�룬��b�����ڶ�����̼ʱ��������̼����������ʱ����ȼ�ŵ�ľ�����ڲ�����b�ˣ����Ϩ����֤�����ˣ����b��
��4��������̼�����������Ʒ�Ӧ����̼��Ƴ����������Թ��ڵ���Һ����ǣ�������̼���뷴Ӧ�����Թ��ڵ�ѹǿ��С��Һ����������������ʯ��ˮ����ǣ��Թ���Һ��������

 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�