��Ŀ����
����Ŀ��ij��Ƭ�ı�ǩ��������֪��Ƭ�ɷ���ֻ��̼����к��и�Ԫ�أ�

��1����2�֣������̼����и�Ԫ�ص���������Ϊ ��
��2����2�֣���ͨ�������ƶϴ˱�ǩ�еĺ������� ������ٻ���ʵ���ģ�
��3����6�֣�ijʵ��С��Ϊ�ⶨ����ʵ�ĺ�������������ʵ�飬ÿ��ȡ10Ƭ��Ƭ�����ѳ����ĺ�����������ձ��У������Ļ�ѧ��Ӧ�ǣ�CaCO3 + 2HCl = CaCl2 + H2O + CO2��,��ַ�Ӧ���ٳ�ȡ�ձ���ʣ�����ʵ����������������£�
���ʵ����� | |
��Ӧǰ���ձ�+���� | 22g |
10Ƭ��Ƭ | 8g |
��Ӧ���ձ�+ʣ���� | 26.7g |
�����˸�Ƭ��̼��Ƶ�����������
![]()
���𰸡���1��40% ��2�� ��� ��3��93.75%
��������
�����������1������Ԫ�ص�������������ı���ʽ����̼����и�Ԫ�ص���������=40/100��100%=40% ��2�����ݱ�ǩ��Ϣ��ÿƿ50Ƭ����40g��ÿƬ��Ƭ������=40g/50=0.8g���ٸ���Ԫ�ص�����=���ʵ�������Ԫ�ص�������������ÿƬ��Ƭ���Ƶ�����=0.8g��40%=0.32g<0.75g������ǩ�еĺ���������� ��3�����ȸ��������غ㶨�ɣ���ѧ��Ӧǰ�����ʵ��������䣬�ʿ������Ӧ���������ɵĶ�����̼����=22g+8g-26.7g=3.3g���ٸ��ݻ�ѧ����ʽ��CaCO3+2HCl==CaCl2+H2O+CO2����CaCO3��CO2��������ϵ����CaCO3�����������������������˸�Ƭ��̼��Ƶ���������
�⣺��CaCO3������Ϊx
CaCO3+2HCl==CaCl2+H2O+CO2��
44
x 3.3g
100��44=x��3.3g
x=7.5g
���˸�Ƭ��̼��Ƶ���������=7.5g/8g��100%=93.75%

����Ŀ����5�֣��ڡ������غ㶨�ɡ��Ŀ��ý�ѧ�У��ס�������ͬѧ�����ˡ���ѧ��Ӧ�У���Ӧ�����������������ϵ����ʵ��̽����ʵ��װ�ú�ѡ��ҩƷ��ͼ��ʾ��
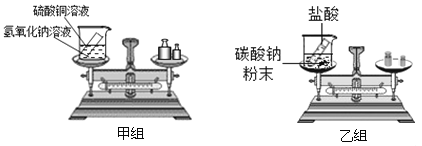
����ʾ��CuSO4+2NaOH==Na2SO4+Cu(OH)2����2HCl+Na2CO3==2NaCl+H2O+CO2����
��1������ʵ��Ŀ�ģ�����ͬѧӦ�ò�����������_____________���ֻ�з�Ӧǰ����ֻ�з�Ӧ����Ӧǰ����֮һ��������Ӧװ�D�������ձ����Թܺ�ҩƷ����������
��2��ʵ����������Ƕ������˹淶�IJ�����ȷ�ij�����ϸ�µĹ۲죺
���� | ���� | |
���� | ������ɫ��������ƽƽ�� | �����غ� |
���� | ���ִ������ݣ���ƽ��ƽ�� | �������غ� |
����ʵ�鷴Ӧ����ƽ��ƽ�⣬��ƽָ���� ƫת������ҡ���������Ϊ������ȷ���� ������顱�����顱������������һ�����۴����ԭ���� ��
��3���������غ㶨�ɿ�֪����ѧ��Ӧǰ��һ��������� ��������ţ�
��ԭ������ ��ԭ����Ŀ ���������� ��������Ŀ ��Ԫ������ ����������