��Ŀ����
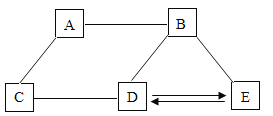
����Ŀ��A��E��Ϊ���л�ѧ����������,����֮��Ĺ�ϵ��ͼ��ʾ(���������Ѿ���ȥ)��
��������ʾ���Ӧ
��������ʾ��Ӧһ��ʵ��
��֪A��Ŀǰ�������������ߵĽ���;B��θ�����Ҫ�ɷ�;C�н���Ԫ�ص���������Ϊ40%,��ˮ��Һ����ɫ,����������ũҩ������Һ;D���ڼ�;E�����Ρ�
��1����C�Ļ�ѧʽΪ_____��
��2��E�׳�_____��ˮ��Һ�����̪��_____ɫ
��3��A��B��Ӧ�Ļ�ѧ����ʽΪ_____��
��4��Eת��ΪD�Ļ�ѧ����ʽΪ_____��
���𰸡�CuSO4 ���� �� Fe+2HCl=FeCl2+H2�� Na2CO3+Ca��OH��2=CaCO3��+2NaOH
��������
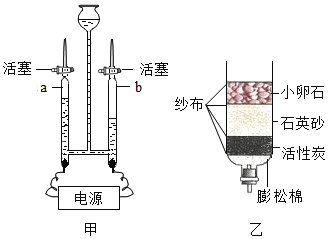
A��E��Ϊ���л�ѧ���������ʣ���֪A��Ŀǰ�������������ߵĽ���������A������B��θ�����Ҫ�ɷ֣�����B�����C�н���Ԫ�ص���������Ϊ40%����ˮ��Һ����ɫ������������ũҩ������Һ������C������ͭ��D���ڼ����ͭ����D������Ӧ������D�������������ƣ�E�����Σ�E���������ƿ����ת��������E��̼���ƣ�������֤���Ƶ���ȷ��
��1��C������ͭ����C�Ļ�ѧʽΪCuSO4��
��2��E��̼���ƣ�E�׳ƴ����ˮ��Һ�Լ��ԣ������̪�Ժ�ɫ��
��3��A��B�ķ�Ӧ���������ᷴӦ�����Ȼ���������������ѧ����ʽΪ��Fe+2HCl=FeCl2+H2����
��4��Eת��ΪD�ķ�Ӧ���������ƺ�̼���Ʒ�Ӧ����̼��Ƴ������������ƣ���ѧ����ʽΪ��Na2CO3+Ca��OH��2=CaCO3��+2NaOH��

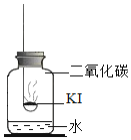
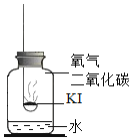
����Ŀ���⻯��![]() ���治������ʡ�ʵ��С���������ʵ��̽��KI���ʵ����ء�
���治������ʡ�ʵ��С���������ʵ��̽��KI���ʵ����ء�
���������1��KI���ʵ�������ʲô��
���������ϣ�![]() Ϊ��ɫ��ĩ����¶�ڿ����л���ûᱻ����Ϊ��
Ϊ��ɫ��ĩ����¶�ڿ����л���ûᱻ����Ϊ��![]() �����Ʊ��ʡ�
�����Ʊ��ʡ�
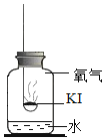
�ڵ�ˮ�к��϶�KIʱ���μӵ�����Һ����ɫ������ɫ
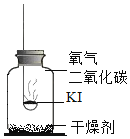
������ʵ�飩�ֱ�ȡ����KI��ȼ�ճ��У��ٷֱ����ʢ�в�ͬ���ʵļ���ƿ�У������������������۲졣
ʵ��1 | ʵ��2 | ʵ��3 | ʵ��4 |
|
|
|
|
����䳱�������� | �������������� | ����䳱���������������� | ����䳱�������� |
����ʵ�飺ȡʵ��1�������ƹ����ܽ⣬���������Һ����Һ����ɫ��ȡʵ��4��������ɫ�����ܽ⣬���������Һ����Һ����ɫ��
����������ۣ�
![]() ʵ��3��Ŀ����______��
ʵ��3��Ŀ����______��
![]() �Ա�ʵ��______�����Եó�KI����һ����ˮ�йء�
�Ա�ʵ��______�����Եó�KI����һ����ˮ�йء�
![]() ������ʵ�����֪��KI���ʵ�������Ҫ��______��
������ʵ�����֪��KI���ʵ�������Ҫ��______��
![]() �Ա�ʵ��1��4�����Գ����ó�����______��KI�����ٶ��йأ����һ������֤��
�Ա�ʵ��1��4�����Գ����ó�����______��KI�����ٶ��йأ����һ������֤��
����˼�����ۣ�
![]() ̽��KI��������ʱ��ͬѧ���ų��˵�����ϡ�������Ӱ�죬��ԭ����______
̽��KI��������ʱ��ͬѧ���ų��˵�����ϡ�������Ӱ�죬��ԭ����______