��Ŀ����
��8�֣�ij��ȤС�黰���У��ڿ����е�ȼþ��ʱ�����������ɵİ�ɫ���������м����������ĵ���ɫ���塣Ϊ��̽����ԭ��С���Ա����������̽�����
[�������] ����ɫ�������ʵijɷ���ʲô��
[��������] ͨ���������ϣ���¼�����м������ʵ���ɫ��
| �� �� | MgO | MgCl2 | Mg3N2 | Mg(NO3)2 | MgCO3 | Mg(OH)2 |
| �� ɫ | ��ɫ | ��ɫ | ����ɫ | ��ɫ | ��ɫ | ��ɫ |
С���Աһ����Ϊ������һ���������Ȼ�þ�������� ��
[�������] ����ɫ�Ĺ�������� �����ѧʽ��
[ʵ��̽��]��1������ͼ��ʾװ���ռ��������ر�a��b��c�������۹���ȼ���ף������ײ���ȼ�ղ���ȴ�����º�a��b��ʹ�ô���Ͳ���г�����A�е�ˮ����Bʱ����c��������������
��2��ʵ����֤�ķ�����
��
[ʵ�����] ��������ȷ�ġ�þ���ڿ�����ȼ�յ��йػ�ѧ��Ӧ����ʽ��
��
[ʵ�鷴˼] ��1������ѡ����ˮ���ռ���ԭ���� ��
��2����̽���������ȼ���µ���ʶ�� ��
��8�֣�[��������]�����в�������Ԫ��
[�������]Mg3N2
[ʵ��̽��]��2����ȼ�ŵ�þ�������1�����ռ��ĵ�����ƿ�У���þ������ȼ�գ����е���ɫ�������ɣ���֤������ɫ������Mg3N2��2�֣�
[ʵ�����] 2Mg+O2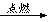 2MgO3Mg+N2
2MgO3Mg+N2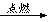 Mg3N2��2�֣�
Mg3N2��2�֣�
[ʵ�鷴˼]��1������������ˮ
��2��ȼ�ղ�һ���������������𰸿ɵ÷֣�
����:��

[�������] ����ɫ�������ʵijɷ���ʲô��
[��������] ͨ���������ϣ���¼�����м������ʵ���ɫ��
| �� �� | MgO | MgCl2 | Mg3N2 | Mg(NO3)2 | MgCO3 | Mg(OH)2 |
| �� ɫ | ��ɫ | ��ɫ | ����ɫ | ��ɫ | ��ɫ | ��ɫ |
[�������] ����ɫ�Ĺ�������� �����ѧʽ��
[ʵ��̽��]��1������ͼ��ʾװ���ռ��������ر�a��b��c�������۹���ȼ���ף������ײ���ȼ�ղ���ȴ�����º�a��b��ʹ�ô���Ͳ���г�����A�е�ˮ����Bʱ����c��������������

��2��ʵ����֤�ķ�����
��
[ʵ�����] ��������ȷ�ġ�þ���ڿ�����ȼ�յ��йػ�ѧ��Ӧ����ʽ��
��
[ʵ�鷴˼] ��1������ѡ����ˮ���ռ���ԭ���� ��
��2����̽���������ȼ���µ���ʶ�� ��
ij��ȤС�黰���У��ڿ����е�ȼþ��ʱ�����������ɵİ�ɫ���������м����������ĵ���ɫ���塣Ϊ��̽����ԭ��С���Ա����������̽�����
[�������]����ɫ�������ʵijɷ���ʲô��
[��������]ͨ���������ϣ���¼�����м������ʵ���ɫ��
|
�� �� |
MgO |
MgCl2 |
Mg3N2 |
Mg(NO3)2 |
MgCO3 |
Mg(OH)2 |
|
�� ɫ |
��ɫ |
��ɫ |
����ɫ |
��ɫ |
��ɫ |
��ɫ |
С���Աһ����Ϊ������һ���������Ȼ�þ�������� ��
[�������]����ɫ�Ĺ�������� �����ѧʽ��
[ʵ��̽��]��1������ͼ��ʾװ���ռ��������ر�a��b��c�������۹���ȼ���ף������ײ���ȼ�ղ���ȴ�����º�a��b��c��ʹ�ô���Ͳ���г�����

��2��Bװ�õ�ȼ���������� ��
��3������ˮ���ռ��õ�����������ʵ������� ��
[ʵ�����]��������ȷ�ġ�þ���ڵ�����ȼ�յ��йػ�ѧ��Ӧ����ʽ��
��
[ʵ�鷴˼]��̽���������ȼ���µ���ʶ�� ��
��8�֣�ij��ȤС�黰���У��ڿ����е�ȼþ��ʱ�����������ɵİ�ɫ���������м����������ĵ���ɫ���塣Ϊ��̽����ԭ��С���Ա����������̽�����
[�������] ����ɫ�������ʵijɷ���ʲô��
[��������] ͨ���������ϣ���¼�����м������ʵ���ɫ��
|
�� �� |
MgO |
MgCl2 |
Mg3N2 |
Mg(NO3)2 |
MgCO3 |
Mg(OH)2 |
|
�� ɫ |
��ɫ |
��ɫ |
����ɫ |
��ɫ |
��ɫ |
��ɫ |
С���Աһ����Ϊ������һ���������Ȼ�þ�������� ��
[�������] ����ɫ�Ĺ�������� �����ѧʽ��
[ʵ��̽��]��1������ͼ��ʾװ���ռ��������ر�a��b��c�������۹���ȼ���ף������ײ���ȼ�ղ���ȴ�����º�a��b��ʹ�ô���Ͳ���г�����A�е�ˮ����Bʱ����c��������������

��2��ʵ����֤�ķ�����
��
[ʵ�����] ��������ȷ�ġ�þ���ڿ�����ȼ�յ��йػ�ѧ��Ӧ����ʽ��
��
[ʵ�鷴˼] ��1������ѡ����ˮ���ռ���ԭ���� ��
��2����̽���������ȼ���µ���ʶ�� ��
ij��ȤС�黰���У��ڿ����е�ȼþ��ʱ�����������ɵİ�ɫ���������м����������ĵ���ɫ���塣Ϊ��̽����ԭ��С���Ա����������̽�����
[�������] ����ɫ�������ʵijɷ���ʲô��
[��������] ͨ���������ϣ���¼�����м������ʵ���ɫ��
| �� �� | MgO | MgCl2 | Mg3N2 | Mg(NO3)2 | MgCO3 | Mg(OH)2 |
| �� ɫ | ��ɫ | ��ɫ | ����ɫ | ��ɫ | ��ɫ | ��ɫ |
С���Աһ����Ϊ������һ���������Ȼ�þ�������� ��
[�������] ����ɫ�Ĺ�������� �����ѧʽ��
[ʵ��̽��]��1������ͼ��ʾװ���ռ��������ر�a��b��c�������۹���ȼ���ף������ײ���ȼ�ղ���ȴ�����º�a��b��ʹ�ô���Ͳ���г�����A�е�ˮ����Bʱ����c��������������

��2��ʵ����֤�ķ�����
��
[ʵ�����] ��������ȷ�ġ�þ���ڿ�����ȼ�յ��йػ�ѧ��Ӧ����ʽ��
��
[ʵ�鷴˼] ��1������ѡ����ˮ���ռ���ԭ���� ��
��2����̽���������ȼ���µ���ʶ�� ��