题目内容
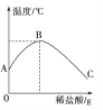
【题目】如图表示的是向盛有10 mL稀的甲溶液(其中滴有少量紫色石蕊试剂)中,加入乙溶液(相同浓度)后,溶液pH的变化曲线。已知甲、乙分别是盐酸和氢氧化钠溶液中的一种。

请分析曲线回答问题:
(1)甲是________溶液;
(2)当加入乙溶液的体积为________mL时,甲、乙溶液恰好完全反应;
(3)当溶液的pH=1.4时,溶液中存在的微观粒子有____________(指示剂除外);
(4)为理解稀盐酸和氢氧化钠两者之间发生反应的微观实质,绘制了如图。请你在右边的圆圈中填入适当的化学用语:______________________

【答案】 NaOH 9 H+、Cl-、Na+ Cl-、Na+、H2O
【解析】由图示可知原甲溶液pH>7,显碱性,加入乙溶液后溶液pH逐渐减小直至pH<7,所以可以断定甲乙溶液,再由图示可知当加入乙的量为9mL时,溶液pH=7;当溶液的pH=1.4时,溶液中的溶质是应该包括生成的物质和剩余的物质;根据复分解反应的实质:发生复分解反应的两种物质在水溶液中相互交换离子,结合成难电离的物质(如水)、难溶的物质(即沉淀)或气体,使溶液中离子浓度降低进行解答。
解:由图示可得:(1)甲溶液显碱性是NaOH溶液,乙溶液显酸性,是盐酸溶液;
(2)当加入乙的量为9mL时,溶液pH=7,甲乙溶液恰好完全反应;
(3)当溶液的pH=1.4时,溶液中的溶质是应该包括生成的物质氯化钠和过量的盐酸。故溶液中存在的微观粒子有:H+、Cl-、Na+;
(4) 根据复分解反应的实质,图中的圆圈中填入适当的化学式或离子符号为:Na+、 Cl-、H2O。

练习册系列答案
相关题目