��Ŀ����
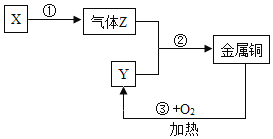
����Ŀ�������٣�W���۵�ߣ������׳���ݵĵ�˿���ú��ٿ���Ҫ�ɷ�����������FeWO4�����۷�ұ�������ٵĹ����������£�
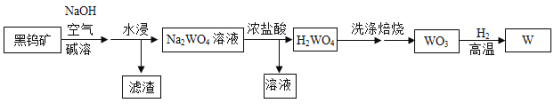
��֪������Na2WO4����ˮ��H2WO4������ˮ��
��1��H2WO4��������___________________��
��2������ǰҪ�����ٿ���飬Ŀ����______________________��
��3��ϴ��H2WO4��Ŀ���dz�ȥNaCl��HCl�ȿ����Ե����ʣ����ȡ���һ�ε�ϴ��Һ�����뼸��__________________��û����������˵��ϴ�Ӹɾ���
��4������ʱ������Ӧ�ķ�Ӧ����Ϊ_______________��
��5��Ũ������Na2WO4��Һ��Ӧ�Ļ�ѧ����ʽΪ_________________��WO3��H2��Ӧ�Ļ�ѧ����ʽΪ____________________��
���𰸡����� �����������ӿ����ʱ�����ķ�Ӧ AgNO3��Һ����������Һ�� �ֽⷴӦ Na2WO4 + 2HCl =2NaCl + H2WO4�� WO3 + 3H2![]() W + 3H2O
W + 3H2O
��������
��1��H2WO4�����������������
��2���ӿ췴Ӧ�����ʣ����Բ��õ�����Ӵ�����������ɽ����ٿ�ʯ��ɷ�ĩ����������������ӿ����ʱ�����ķ�Ӧ��
��3�����������������ӽ�ϰ�ɫ����������ϴ��H2WO4��Ŀ���dz�ȥNaCl��HCl�ȿ����Ե����ʣ����ȡ���һ�ε�ϴ��Һ�����뼸����������Һ��û����������˵��ϴ�Ӹɾ������AgNO3��Һ��
��4������ʱ��һ���������������������ʣ����ڷֽⷴӦ������ֽⷴӦ��
��5��Ũ������Na2WO4��Һ��Ӧ�����Ȼ��ƺ�����������ڸ��µ������£�WO3��H2��Ӧ���ɽ����ٺ�ˮ�����Na2WO4+2HCl�T2NaCl+H2WO4����WO3+3H2![]() W+3H2O��
W+3H2O��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ�����ݵ���ʯ��ʯ��Դ�ḻ��ij��ѧ��ȤС���ȡ4.0gʯ��ʯ��Ʒ����40gϡ�����4�μ�����Ʒ��(�������ʲ���ӦҲ���ܽ�)����ʵ���������£�
ϡ��������� | ʣ���������� |
��һ�μ���10g | 3.0g |
�ڶ��μ���10g | 2.0g |
�������10g | 1.0g |
���Ĵμ���10g | 0.6g |
����㣺
(1)4.0gʯ��ʯ��Ʒ��̼��Ƶ�������____g��
(2)10gϡ��������_____g̼���������ȫ��Ӧ��
(3)��ϡ�����������������Ϊ_________(д��������̣������ȷ��0.1%)