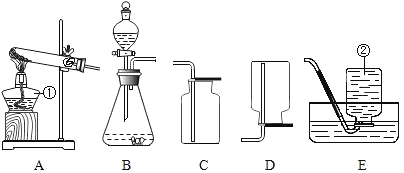
��Ŀ����
�±���NaCl��KNO3�ڲ�ͬ�¶�ʱ���ܽ�ȣ��������ݻش�
�¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 | 60 | |
�ܽ�� | NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 |
��g/100gˮ�� | KNO3 | 13.3 | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110 |
���Ȼ��Ƶ��ܽ�����¶ȱ仯��Ӱ��_____����ܴ�С������
��50��ʱ��KNO3���ܽ��_____���������������������NaCl���ܽ�ȣ����ձ��м���100gˮ��49.0gKNO3�������50�����Һ������ȴ��20�棬�ձ����������������Ϊ_____g��
��KNO3�л���������NaCl���ᴿ�ķ�����_____������½ᾧ���������ᾧ������
��Ҫ�Ƚ�NaCl��KNO3��ˮ�е��ܽ���ǿ�����ⶨ�����ݿ����ǣ���ͬ�¶��£���������������ȫ�ܽ�ﵽ����ʱ����ˮ����������_____��
����ͼ���й��������Һ��ʵ��������仯�����˵����ȷ����_____�����ţ���
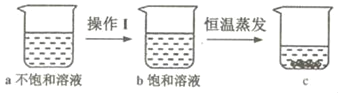
A ����Iһ���ǽ���
B a��b���ܼ������������
C a��c����������һ�����
D b��c��������������һ�����
��ϰ��ϵ�д�
�����Ŀ


 Si+2CO������ȡ�ֹ裬����˵����ȷ����
Si+2CO������ȡ�ֹ裬����˵����ȷ����