��Ŀ����
ijͬѧ������������ͭ��Һ��Ӧ��ʵ��ʱ����������ͭ��ͬʱ�����ݲ��������ǻ�������ͭ��Һ������Ϊ��֤ʵ���룬��������ʵ�飺
a��ȡ8.5g���۷���100g������ͭ��Һ�У���Ӧ��ɺ��˳����壬ϴ�ӡ�������������������Ϊ9.2g��
b����9.2g������������ϡ�����ַ�Ӧ��Ӧ����������������Ϊ0.1g�����������������________g��
���㣺
��1������ͭ��Һ����������������
��2������ʵ������������ͭ��Һ��ϡ���ᷴӦ��������________g���Դν��������������________��
�⣺b����9.2g������������ϡ���ᷴӦ����������Ϊx
Fe+H2SO4=FeSO4+H2��
56 2
x 0.1g
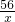 =
=
��ã�x=2.8g��
���������������2.8g��
�ʴ�Ϊ��2.8��
��1��9.2g������ͭ������Ϊ��9.2g-2.8g=6.4g
��������Ӧ������ͭ������Ϊy����������Ϊz
Fe+CuSO4=FeSO4+Cu
56 160 64
z y 6.4g
 =
= ��
�� =
= ��
��
��ã�y=16g��z=5.6g��
������ͭ��Һ��������������Ϊ ��100%=16%��
��100%=16%��
������ͭ��Һ��������������Ϊ16%��
��2��������Ľ���֪����ϡ���ᷴӦ����������Ϊ2.8g��������ͭ��Ӧ����������Ϊ5.6g��
����������ͭ��Һ��ϡ���ᷴӦ��������Ϊ��2.8g+5.6g=8.4g��8.5g��
��Ϊ��������������ͭ��Һ�е��������ʷ�Ӧ���������壮
�ʴ�Ϊ��С��8.5��Ϊ8.4����Ϊ��������������ͭ��Һ�е��������ʷ�Ӧ���������壮
������b����������ϡ���ᷴӦ�Ļ�ѧ����ʽ�������������������������������Ϊ������ٵ�������
��1��9.2g��������ϡ���ᷢ����Ӧ��˵��9.2g���岻ȫ��ͭ������������9.2g-�����л��е���������=ͭ��������������������ͭ��Ӧ�Ļ�ѧ����ʽ������ͭ����������Ӧ������ͭ���������ٸ�������ͭ��Һ�����������������ͭ��Һ����������������
��2��������������ͭ��Һ��Ӧ��ʵ��ʱ����������ͭ��ͬʱ�����ݲ�����˵������ͭ��Һ�л����ᣬ������������ͭ��Һ�е��ᷢ����Ӧ�����Ի����������ͭ��Ӧ��������������ϡ���ᷴӦ����������֮��С��8.5g��
������������ͭ������Ӧ��ʵ��ʱ�����ɵ�ͭ���������ı��棬�������������ȫ��Ӧ�����Խ����ɵ�ͭ���������л����������ɣ�����ʵ��δ��Ӧ��������ϡ���ᷢ����Ӧ��
Fe+H2SO4=FeSO4+H2��
56 2
x 0.1g
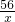 =
=
��ã�x=2.8g��
���������������2.8g��
�ʴ�Ϊ��2.8��
��1��9.2g������ͭ������Ϊ��9.2g-2.8g=6.4g
��������Ӧ������ͭ������Ϊy����������Ϊz
Fe+CuSO4=FeSO4+Cu
56 160 64
z y 6.4g
 =
= ��
�� =
= ��
����ã�y=16g��z=5.6g��
������ͭ��Һ��������������Ϊ
 ��100%=16%��
��100%=16%��������ͭ��Һ��������������Ϊ16%��
��2��������Ľ���֪����ϡ���ᷴӦ����������Ϊ2.8g��������ͭ��Ӧ����������Ϊ5.6g��
����������ͭ��Һ��ϡ���ᷴӦ��������Ϊ��2.8g+5.6g=8.4g��8.5g��
��Ϊ��������������ͭ��Һ�е��������ʷ�Ӧ���������壮
�ʴ�Ϊ��С��8.5��Ϊ8.4����Ϊ��������������ͭ��Һ�е��������ʷ�Ӧ���������壮
������b����������ϡ���ᷴӦ�Ļ�ѧ����ʽ�������������������������������Ϊ������ٵ�������
��1��9.2g��������ϡ���ᷢ����Ӧ��˵��9.2g���岻ȫ��ͭ������������9.2g-�����л��е���������=ͭ��������������������ͭ��Ӧ�Ļ�ѧ����ʽ������ͭ����������Ӧ������ͭ���������ٸ�������ͭ��Һ�����������������ͭ��Һ����������������
��2��������������ͭ��Һ��Ӧ��ʵ��ʱ����������ͭ��ͬʱ�����ݲ�����˵������ͭ��Һ�л����ᣬ������������ͭ��Һ�е��ᷢ����Ӧ�����Ի����������ͭ��Ӧ��������������ϡ���ᷴӦ����������֮��С��8.5g��
������������ͭ������Ӧ��ʵ��ʱ�����ɵ�ͭ���������ı��棬�������������ȫ��Ӧ�����Խ����ɵ�ͭ���������л����������ɣ�����ʵ��δ��Ӧ��������ϡ���ᷢ����Ӧ��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ