��Ŀ����
��2012?����ģ�⣩С��ͬѧ����ѧ�IJ��ֻ�ѧ֪ʶ�������£����в���ȫ��ȷ���ǣ�������
|
������A�����ݲ��ϵķ����Լ�ë֯Ʒȼ�����ս���ë��ζ�����н��
B������ʳƷ��ȫ�ij�ʶ���н��
C�����ݵ�̼���õ�Ҫ����н��
D���������ʵ����ʽ��н��
B������ʳƷ��ȫ�ij�ʶ���н��
C�����ݵ�̼���õ�Ҫ����н��
D���������ʵ����ʽ��н��
����⣺A��ë֯Ʒȼ�����ս���ë��ζ����ë֯Ʒ����֯Ʒ������-ȼ�պ�����ζ���ֽ��������������̥-���ϲ��ϣ��������մɡ�ˮ��-���ǽ������ϣ���A��ȷ��
B���״��ж����������ã������ܻ�����ʳ����ϲ���ʳ�ã�����ù��-�����л��ù��ʳ�ﲻ��ʳ�ã����շ���֢����B��ȷ��
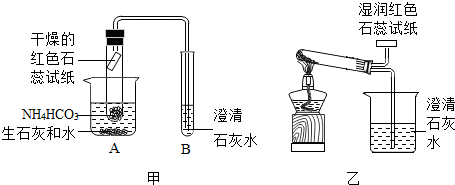
C���������̭�ߺ��ܡ�����Ⱦ��ҵ����������Դ���洫ͳ��Դ������ʹ��һ���Կ��ӣ������ڼ��ٶ�����̼���ŷţ����ϵ�̼���õ�Ҫ��C��ȷ��
D������ʳ�;ƾ�-����ζ����������֧��ȼ�գ�˵������������ȼ�ԣ���ȼ����Ż�������ʵ����ԣ����ܽ��ͣ���D����
��ѡ��D��
B���״��ж����������ã������ܻ�����ʳ����ϲ���ʳ�ã�����ù��-�����л��ù��ʳ�ﲻ��ʳ�ã����շ���֢����B��ȷ��
C���������̭�ߺ��ܡ�����Ⱦ��ҵ����������Դ���洫ͳ��Դ������ʹ��һ���Կ��ӣ������ڼ��ٶ�����̼���ŷţ����ϵ�̼���õ�Ҫ��C��ȷ��
D������ʳ�;ƾ�-����ζ����������֧��ȼ�գ�˵������������ȼ�ԣ���ȼ����Ż�������ʵ����ԣ����ܽ��ͣ���D����
��ѡ��D��
������ѧ��Դ�����������Ҳ�����������������������������������йصĻ�ѧ֪ʶ��������ȵ�֮һ��

��ϰ��ϵ�д�
 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д� ����������������ϵ�д�
����������������ϵ�д�
�����Ŀ