��Ŀ����
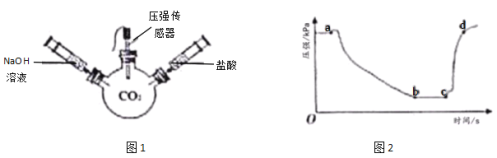
����Ŀ��ʯ��ʯ����Ҫ�ɷ�ΪCaCO3���������ʲ�����ˮ����μӷ�Ӧ����ij��ѧ��ȤС��Ϊ�˲ⶨʯ��ʯ��CaCO3����������������������̽��ʵ�飺��ʯ��ʯ��Ʒ���ݣ��ֱ������ձ��У���һ���ձ��м���50��ϡ���ᣬ�ڶ����ձ��з���100��ϡ���ᣬ��ַ�Ӧ�����������ݲ���ʱ�����������������й��������±�����1�������ձ���CO2����������2��ʯ��ʯ��Ʒ��CaCO3��������������3��ϡ���������ʵ�����������
�����ʵ����� | ��һ���ձ� | �ڶ����ձ� |
��Ʒ���� | 16g | 16 g |
ϡ�������� | 50g | 100g |
�ձ����������� | 61.6 g | 109.4g |
������CO2���� |
���𰸡���1��4.4g��6.6g����2��93.8%����3��14.6%��
��������
�������ȷ�����������û�ѧ����ʽ��̼��ƺ�ϡ����ķ�ӦCaCO3+2HCl==CaCl2+H2O+CO2����
��1�����������غ㶨�ɣ���Ӧ�����ɵĶ�����̼�����Ƿ�Ӧǰ���ձ������������IJ�ֵ��
��һ���ձ��У����ɶ�����̼������=16g+50g-61.6g=4.4g��
�ڶ����ձ��У����ɶ�����̼������=16g+100g-109.4g=6.6g
��2�����������ձ������ɶ�����̼�������������ڶ����ձ���������ʣ�̼࣬���ȫ����Ӧ����˸��ݵڶ����ձ��ж�����̼���������ʯ��ʯ��̼��Ƶ�������Ȼ�����̼��Ƶ�����������
��16gʯ��ʯ��̼��Ƶ�����Ϊx��
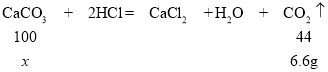
![]()
���x=15g
ʯ��ʯ��̼��Ƶ���������=![]()
��3�����������ձ������ɶ�����̼��������������һ���ձ���ʯ��ʯ��ʣ�࣬����ȫ����Ӧ����˸��ݵ�һ���ձ��ж�����̼������������������ʵ�������Ȼ��������������������
��50g���������ʵ�����Ϊy��
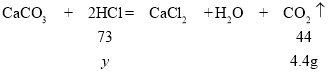
![]()
���y=7.3g
ϡ�����������������Ϊ��![]() ��
��

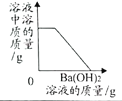
����Ŀ������ͼ��ֱ��ʾ4��ʵ��Ĺ����У�����ͼ�����Ӧʵ������ϵ���
|
|
|
|
A.��һ������ϡH2SO4�м���Ba(OH)2��ǡ���к� | B.��һ�������IJ�����KNO3��Һ�����������о������� | C.��������Ľ���Fe��Zn�зֱ�����Ũ�ȵ�ϡ���� | D.CuO��C�Ĺ��������ڸ����·�Ӧ |
A. AB. BC. CD. D