��Ŀ����
ijʵ��С������йغ���ȼ�յ�ϵ��ʵ��(��ͼ)��
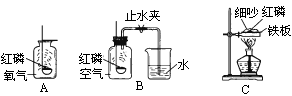
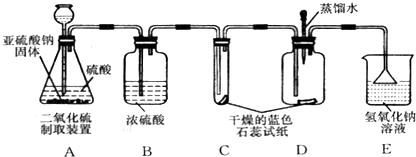
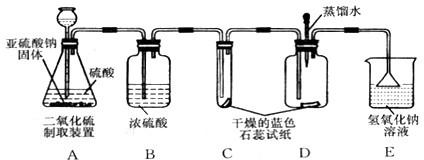
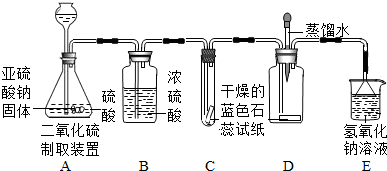
(1)ʵ��һ����ͼA��ʾ������ȼ�ĺ��ײ��뼯��ƿ�У������������о���ȼ�գ��ɿ�������ƿ�в�������________���÷�Ӧ�����ֱ���ʽΪ________���������Ӧ������________��Ӧ��
(2)ʵ�������ͼB��ʾ������������ȼ���뼯��ƿ�У�������Ƥ������ȼ��ֹͣ����ȴ�����º�ֹˮ�У��ɹ۲쵽�ձ��е�ˮ����������ƿ�ڣ�����ˮ�������Լռԭƿ�ڿ��������![]() ����ʵ��Ŀ����________________��
����ʵ��Ŀ����________________��
(3)ʵ��������ͼC��ʾ����������������һ�������ϣ�����ϸɰ��ȫ���ǣ��þƾ��Ƽ�������һ��ʱ�䣬��������������������Ϊ________����ȥ�ƾ��ƺ�������ɰ�Ӳ���¶�����ף���������ȼ�գ�������Ϊ________��
�𰸣�
������
������
|
����(1)����,�ף����� ����(2)�ⶨ�����������ĺ���(��֤�����������Լռ���������1��5��) ����(3)����û���������Ӵ������Ժ��ײ�ȼ��,����ϸɳ������������Ӵ������¶ȴﵽ����ȼ�����������¶ȣ����Ժ���ȼ�� |

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ