��Ŀ����
��2008?�ϸ�����ģ��ʵ������һƿŨ���ᣮƿ�ϱ�ǩ��������ͼ��ʾ������ݱ�ǩ���ṩ�����ݽ���������⣺��1����Ҫ������80g NaoH�ķ�Һ����ѡ����ͼ������Ũ�����кͣ�����Ũ���������Ϊ______��
��2��98g10%�����lOgij�Ͻ𣨺Ͻ�����һ��δ֪������M����ͭ��ɵģ���Ϻ��ַ�Ӧ�����ˣ��õ���������Һ����������������Ϊ7.6g����δ֪������Ӧ���γɻ�����Ļ��ϼ�Ϊ+2�ۣ��������м����ᣮ�������ܽ⣬����Һ�мӺϽ�Ҳû���κ�����
��д��������Ӧ�Ļ�ѧ����ʽ______
��������֪������ⷴӦ��������������x���ı���ʽ______
�۽���M��Ԫ�ط���Ϊ______
�ܺϽ���ͭԪ�ص���������Ϊ______
��������Һ���ʵ���������______��

���𰸡���������1�������������ƺ����ᷴӦ�Ļ�ѧ����ʽ�Լ��������Ƶ��������г�����ʽ���Ϳ��������Ũ�����������
��2���������⡰�Ͻ�����һ��δ֪������M����ͭ��ɵġ���֪�������ᷴӦ��ֻ�н���M�����ҡ�����δ֪������Ӧ���γɻ�����Ļ��ϼ�Ϊ+2�ۡ����ɴ�д������ʽ��
�ڸ���M�����ᷴӦ�Ļ�ѧ����ʽ�����ɵó���ⷴӦ��������������x���ı���ʽ��
�۸���M�����ᷴӦ�Ļ�ѧ����ʽ�������������M�����ԭ������������ԭ����������֪��ʲô������д����Ԫ�ط��ż��ɣ�
����֪��������������������Ϊ7.6g�����ǺϽ���ͭ����������������������ʽ���㼴�ɣ�
�ݸ���M�����ᷴӦ�Ļ�ѧ����ʽ�������غ㶨�ɣ����ɼ������Һ�����ʵ�������Ȼ�������������������ʽ���㼴�ɣ�
����⣺��1��������Ũ���������Ϊy��
2NaOH+H2SO4=Na2SO4+2H2O
80 98
80g 98%x
��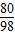 =
=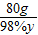
��֮�ã�y=100g��
�ʴ�Ϊ��100g��
��2����M�����ᷴӦ�ķ���ʽΪ��M+H2SO4=MSO4+H2����
���跴Ӧ������������Ϊx��M�����ԭ������Ϊz��
M+H2SO4=MSO4+H2��
z 98 2
10g-7.6g 98g×10% x
��98��2=��98g×10% ����x��z��98=��10g-7.6g ������98g×10%��
��֮�ã�x=0.2g��z=24��
���ɢ��м������M�����ԭ������Ϊ24��֪���˽�����þ���������ΪMg��
�ܺϽ���ͭԪ�ص���������Ϊ��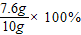 =76%��
=76%��
�ݸ��������غ㶨�ɣ���Ӧ���ɵ�MgSO4������Ϊ����10g-7.6g��+��98g×10%��-0.2g=12g��
������Һ������Ϊ��98g+10g-7.6g-0.2g=100.2g
������Һ���ʵ���������Ϊ��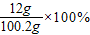 ��12.0%��
��12.0%��
�ʴ�Ϊ����M+H2SO4=MSO4+H2������98��2=��98g×10% ����x����Mg����76%����12.0%��
������������Ҫ����ѧ�����������غ㶨�ɺͻ�ѧ����ʽ�Լ����ʵ�����������ʽ���м����������
��2���������⡰�Ͻ�����һ��δ֪������M����ͭ��ɵġ���֪�������ᷴӦ��ֻ�н���M�����ҡ�����δ֪������Ӧ���γɻ�����Ļ��ϼ�Ϊ+2�ۡ����ɴ�д������ʽ��
�ڸ���M�����ᷴӦ�Ļ�ѧ����ʽ�����ɵó���ⷴӦ��������������x���ı���ʽ��
�۸���M�����ᷴӦ�Ļ�ѧ����ʽ�������������M�����ԭ������������ԭ����������֪��ʲô������д����Ԫ�ط��ż��ɣ�
����֪��������������������Ϊ7.6g�����ǺϽ���ͭ����������������������ʽ���㼴�ɣ�
�ݸ���M�����ᷴӦ�Ļ�ѧ����ʽ�������غ㶨�ɣ����ɼ������Һ�����ʵ�������Ȼ�������������������ʽ���㼴�ɣ�
����⣺��1��������Ũ���������Ϊy��
2NaOH+H2SO4=Na2SO4+2H2O
80 98
80g 98%x
��
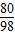 =
=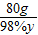
��֮�ã�y=100g��
�ʴ�Ϊ��100g��
��2����M�����ᷴӦ�ķ���ʽΪ��M+H2SO4=MSO4+H2����
���跴Ӧ������������Ϊx��M�����ԭ������Ϊz��
M+H2SO4=MSO4+H2��
z 98 2
10g-7.6g 98g×10% x
��98��2=��98g×10% ����x��z��98=��10g-7.6g ������98g×10%��
��֮�ã�x=0.2g��z=24��
���ɢ��м������M�����ԭ������Ϊ24��֪���˽�����þ���������ΪMg��
�ܺϽ���ͭԪ�ص���������Ϊ��
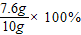 =76%��
=76%���ݸ��������غ㶨�ɣ���Ӧ���ɵ�MgSO4������Ϊ����10g-7.6g��+��98g×10%��-0.2g=12g��
������Һ������Ϊ��98g+10g-7.6g-0.2g=100.2g
������Һ���ʵ���������Ϊ��
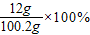 ��12.0%��
��12.0%���ʴ�Ϊ����M+H2SO4=MSO4+H2������98��2=��98g×10% ����x����Mg����76%����12.0%��
������������Ҫ����ѧ�����������غ㶨�ɺͻ�ѧ����ʽ�Լ����ʵ�����������ʽ���м����������

��ϰ��ϵ�д�
 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д� Ӣ�żƻ���ĩ����ϵ�д�
Ӣ�żƻ���ĩ����ϵ�д�
�����Ŀ