��Ŀ����
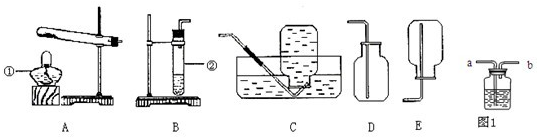
ʵ������˫��ˮ��������̷�ĩ�������������1����Ӧ�ķ���װ�ú��ռ�װ�÷ֱ�ѡ���ͼ�е�______��
��2��ʵ�����ṩ�Ĺ���������Һ��ǩ����ͼ���������ϵ�֪��������������10%��˫��ˮ���ʺ���ȡ��������100��������������30%��˫��ˮ�����ó�������������10%��˫��ˮ���ˮ______������
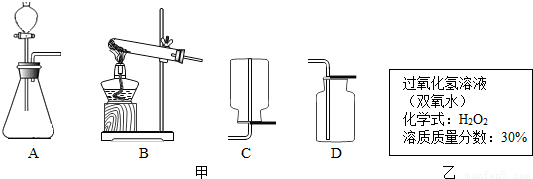
��3��ȡ30��ϡ�ͺ��˫��ˮ�������Ķ������̷�ĩ��ϣ����ݻ�ѧ����ʽ���㣬�����Ͽ��Ƶ��������ٿˣ����������һλС����
���𰸡���������1�����ݷ���װ�õ�ѡ�����ǣ���Ӧ��Ϊ������ȡ����ʱ��Ҫ���ȣ���Ӧ���ǹ����Һ����ȡ����ʱ������Ҫ���ȣ��������������ʷ����ռ�װ�ã�
��2��������Һϡ��������������������н��
��3�����ݻ�ѧ����ʽ���ù����������������������������ɣ�
����⣺��1����˫��ˮ��������̷�ĩ����������ǹ����Һ����ȡ����ʱ������Ҫ���ȣ����Է���װ��ѡA���������ܶȴ��ڿ������ܶȣ������ռ�װ��ѡ��D�����AD��
��2������Ҫ��ˮ������Ϊx��100g×30%=��100g+x��×10%�����x=200g������ˮ���ˮ200���������200��
��3���������Ͽ��Ƶ�����������Ϊx��
2H2O2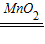 2H2O+O2��
2H2O+O2��
68 32
30g×10% x
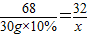
x=1.4g
�������Ͽ��Ƶ�����������Ϊ1.4g��
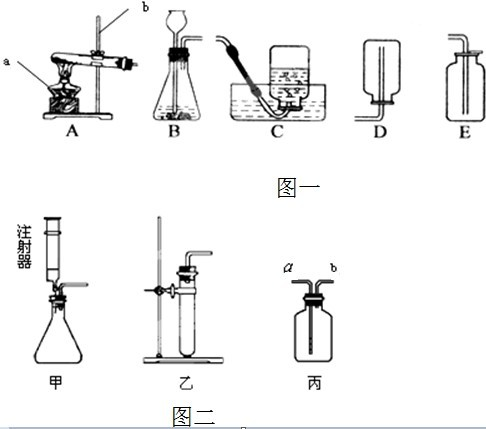
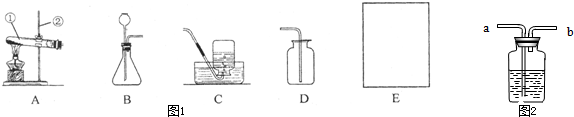
��������������ʵ��������ȡ����ķ���װ�ú��ռ�װ�õ�ѡ�����ݣ�����������ȷ��ѡ��ס����������ȡ�ķ�Ӧԭ�������ܹ���ȷ��д��ѧ����ʽ��
��2��������Һϡ��������������������н��
��3�����ݻ�ѧ����ʽ���ù����������������������������ɣ�
����⣺��1����˫��ˮ��������̷�ĩ����������ǹ����Һ����ȡ����ʱ������Ҫ���ȣ����Է���װ��ѡA���������ܶȴ��ڿ������ܶȣ������ռ�װ��ѡ��D�����AD��
��2������Ҫ��ˮ������Ϊx��100g×30%=��100g+x��×10%�����x=200g������ˮ���ˮ200���������200��
��3���������Ͽ��Ƶ�����������Ϊx��
2H2O2
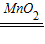 2H2O+O2��
2H2O+O2��68 32
30g×10% x
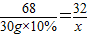
x=1.4g
�������Ͽ��Ƶ�����������Ϊ1.4g��
��������������ʵ��������ȡ����ķ���װ�ú��ռ�װ�õ�ѡ�����ݣ�����������ȷ��ѡ��ס����������ȡ�ķ�Ӧԭ�������ܹ���ȷ��д��ѧ����ʽ��

��ϰ��ϵ�д�
 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�
�����Ŀ
 26��
26��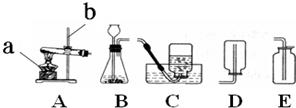 ʵ���ҳ�������װ������ȡ������
ʵ���ҳ�������װ������ȡ������