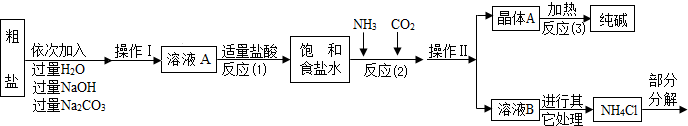
��Ŀ����
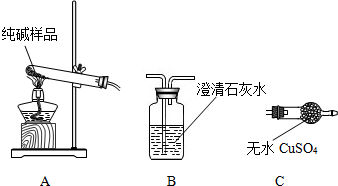
 30����1����ͼ��ʾ��ij������庬�г��л�ѧ���������壬��������ͨ��װ�г����ʯ��ˮ�ļ��������Լ�ƿ�����������������еij����ʯ��ˮ����ǣ��������ijɷ�Ϊ
30����1����ͼ��ʾ��ij������庬�г��л�ѧ���������壬��������ͨ��װ�г����ʯ��ˮ�ļ��������Լ�ƿ�����������������еij����ʯ��ˮ����ǣ��������ijɷ�ΪHCl��CO2
�����з�����Ӧ�Ļ�ѧ����ʽΪCa��OH��2+2HCl=CaCl2+2H2O
����2������ϡ���ᡢʯ��ˮ��ʳ��ˮ��̼������Һ������ɫ��Һ��Ϊ�������Ƿֱ���ΪA��B��C��D��Ȼ���ȡ���������������ʵ�飬������ұ���ͼ�С�������������-�����ŷֱ��ʾ���ɳ���������������Ա仯�������ݱ���ʵ�������ж�
| A | B | C | D | |
| A | - | - | �� | - |
| B | - | - | �� | - |
| C | �� | �� | - | - |
| D | - | - | - | - |
HCl
���ѧʽ������A��C������Ӧ�Ļ�ѧ����ʽΪ
Na2CO3+Ca��OH��2=CaCO3��+2NaOH
���÷�Ӧ�Ļ�����Ӧ���������ֽⷴӦ
����3��ij����ѧϰС����̼������Һ�ֱ�������ʯ��ˮ�����ᣮʵ���Һ����ͬһ�ձ��У�����ձ���Һ����壮��ʦ��������С���ͬѧ���۷�������Ϊ���ձ��г������Һ���ܻẬ���������ʣ�Na2CO3��HCl��Ca��OH��2��NaOH��NaCl��CaCl2��������ݳ�����ѧ��ѧ֪ʶ�жϣ����ձ�������Һ��һ�����е�������
NaCl��CaCl2
��һ�������е�������Ca��OH��2��Na2CO3��NaOH
����������1�����ݼ��������������еij����ʯ��ˮ����ǣ��жϻ������ijɷ֣�
��2�����ݱ�����A��C��Ӧ���ɳ�����ȷ��A��C��ʯ��ˮ��̼������Һ������B��C��Ӧ�������壬C��̼������Һ��B��ϡ���ȷ�������ʺ�д��A��C������Ӧ�Ļ�ѧ����ʽ���Լ���Ӧ���ͣ�
��3���������ַ�Ӧ�ķ�Ӧ��������Լ�����֮��ķ�Ӧ�жϣ�
��2�����ݱ�����A��C��Ӧ���ɳ�����ȷ��A��C��ʯ��ˮ��̼������Һ������B��C��Ӧ�������壬C��̼������Һ��B��ϡ���ȷ�������ʺ�д��A��C������Ӧ�Ļ�ѧ����ʽ���Լ���Ӧ���ͣ�
��3���������ַ�Ӧ�ķ�Ӧ��������Լ�����֮��ķ�Ӧ�жϣ�
����⣺��1���������ijɷ���HCl��CO2����Ҫ���Ȼ����к����������ƣ����¶�����̼�����������Ʒ�Ӧ���ɳ��������Լ��������������еij����ʯ��ˮ����ǣ����з�����Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+2HCl=CaCl2+2H2O��
�ʴ�Ϊ��HCl��CO2��Ca��OH��2+2HCl=CaCl2+2H2O��
��2����A��C��Ӧ���ɳ�����ȷ��A��C��ʯ��ˮ��̼������Һ������B��C��Ӧ�������壬C��̼������Һ��B��ϡ���A��ʯ��ˮ��D��ʳ��ˮ��
�ʴ�Ϊ��HCl��
�ڸ���A��ʯ��ˮ��C��̼������Һ�����߷�Ӧ�Ļ�ѧʽ�ǣ�Na2CO3+Ca��OH��2=CaCO3��+2NaOH�������ֻ�����������ɷ֣������������ֻ�����ĸ��ֽⷴӦ��
�ʴ�Ϊ��Na2CO3+Ca��OH��2=CaCO3��+2NaOH�����ֽⷴӦ��
��3������̼������Һ��ʯ��ˮ�Ļ�ѧ��Ӧʽ�ǣ�Na2CO3+Ca��OH��2�T2NaOH+CaCO3����̼������Һ��ϡ����Ļ�ѧ��Ӧʽ�ǣ�Na2CO3+2HCl�T2NaCl+H2O+CO2����Na2CO3��HCl��Ca��OH��2��NaOH��NaCl��CaCl2��NaCl����������Ҳ����������ʷ�Ӧ������һ���У�ʵ���Һ����ͬһ�ձ��У�����ձ���Һ����壮˵�����ɵij���CaCO3���������ᷴӦ�ˣ���������CaCl2�����������������ʷ�Ӧ������Ҳһ���У�Ca��OH��2��Na2CO3��NaOH���������ᷴӦ������һ��û�У�
�ʴ�Ϊ��NaCl��CaCl2��Ca��OH��2��Na2CO3��NaOH��
�ʴ�Ϊ��HCl��CO2��Ca��OH��2+2HCl=CaCl2+2H2O��
��2����A��C��Ӧ���ɳ�����ȷ��A��C��ʯ��ˮ��̼������Һ������B��C��Ӧ�������壬C��̼������Һ��B��ϡ���A��ʯ��ˮ��D��ʳ��ˮ��
�ʴ�Ϊ��HCl��
�ڸ���A��ʯ��ˮ��C��̼������Һ�����߷�Ӧ�Ļ�ѧʽ�ǣ�Na2CO3+Ca��OH��2=CaCO3��+2NaOH�������ֻ�����������ɷ֣������������ֻ�����ĸ��ֽⷴӦ��
�ʴ�Ϊ��Na2CO3+Ca��OH��2=CaCO3��+2NaOH�����ֽⷴӦ��
��3������̼������Һ��ʯ��ˮ�Ļ�ѧ��Ӧʽ�ǣ�Na2CO3+Ca��OH��2�T2NaOH+CaCO3����̼������Һ��ϡ����Ļ�ѧ��Ӧʽ�ǣ�Na2CO3+2HCl�T2NaCl+H2O+CO2����Na2CO3��HCl��Ca��OH��2��NaOH��NaCl��CaCl2��NaCl����������Ҳ����������ʷ�Ӧ������һ���У�ʵ���Һ����ͬһ�ձ��У�����ձ���Һ����壮˵�����ɵij���CaCO3���������ᷴӦ�ˣ���������CaCl2�����������������ʷ�Ӧ������Ҳһ���У�Ca��OH��2��Na2CO3��NaOH���������ᷴӦ������һ��û�У�
�ʴ�Ϊ��NaCl��CaCl2��Ca��OH��2��Na2CO3��NaOH��
���������⿼����������ж����ʣ�Ҫͬѧ�Ƕ���ѧ���ʵ����ʡ�״̬�����������ʵķ�Ӧ����Ҫ��Ȼ���ģ�����������ԣ��ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ