��Ŀ����
��ˮ���̺�80����Ԫ�أ���һ����ı��ء���1����ˮɹ�������� ��������ᾧ������ȴ�ᾧ�����ķ�����
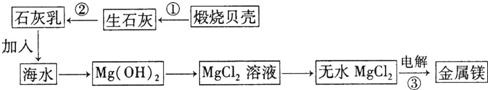
��2��þ�Ͻ��ǿ�ȸߡ���е���ܺã���ɷ�þ�������������ɻ����������Ҫ���ϣ����С���������������������ͼ�ǴӺ�ˮ����ȡþ��ȫ���̣���ش��������⣺

�� þ�Ͻ��� ������ʡ������������������ ��
����ͼ��þԪ�ص�ij�����ӵĽṹʾ��ͼ����ͼ��ʾ �������ӷ��ţ���

�� ��ѧ��Ӧǰ����Ԫ�ػ��ϼ۱仯�ķ�Ӧ����������ԭ��Ӧ�����Тۢݵķ�Ӧ����������ԭ��Ӧ���� ���仯ѧ����ʽΪ �����ڸ��ֽⷴӦ���� ���仯ѧ����ʽΪ ��
��1�������ᾧ
��2���ٻ�����Mg2+(3����MgCl2 Mg+Cl2�� �� Mg��OH��2+2HCl= MgCl2+2H2O����:
Mg+Cl2�� �� Mg��OH��2+2HCl= MgCl2+2H2O����:
��
��2���ٻ�����Mg2+(3����MgCl2
 Mg+Cl2�� �� Mg��OH��2+2HCl= MgCl2+2H2O����:
Mg+Cl2�� �� Mg��OH��2+2HCl= MgCl2+2H2O����:��

��ϰ��ϵ�д�
 �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
���ƿ�����ϵ�д� ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д�
�����Ŀ