��Ŀ����
����Ŀ��ʵ������һ�ݲ��ֱ�����������þ��þ����Ʒ��δ֪Ũ�������40%������������Һ����ȤС��ͬѧΪ������Ʒ�н���þ���������������ͼ��������������������������̽����
��1���������ܽ���Ʒ����Ʒ��Ͼ��ȣ����ⶨ��������������ʵ�����������ʾ��
ʵ����� | ��ȡ��Ʒ������g�� | ��������������g�� | ��������������g�� |
�� | 16.0 | 60.0 | 0.5 |
�� | 16.0 | 130.0 | 1.0 |
�� | 16.0 | 150.0 | 1.0 |
þ����Ʒ�н���þ�������ٷֺ���Ϊ ��
��2����ʵ�������Һ�м���40%������������Һ�����ɳ������������������������Һ�����ı仯��ϵ��ͼ��ʾ�����������������������д��������̣� 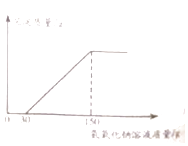
���𰸡�
��1��75.0%
��2���⣺MgO+2HCl=MgCl2+H2O��Mg+2HCl=MgCl2+H2����MgCl2+2NaOH=Mg��OH��2��+2NaCl��HCl+NaOH=NaCl+H2O���ɵù�ϵʽ��HCl��NaOH����150.0gϡ�������Ȼ��������Ϊy
HCl�� | NaOH |
36.5 | 40 |
y | 150g��40% |
![]()
y=54.75g
�������������Ϊ ![]() =36.5%
=36.5%
���������������Ϊ36.5%
���������⣺��1���Աȵڢ����з��֣��Աȵڢ�������������������130g���ӵ�150gʱ������������û�����ӣ�˵���ڢ�����þ��ȫ��Ӧ��
����Ʒ�н���þ������Ϊx��
Mg+2HCl=MgCl2+ | H2�� |
24 | 2 |
x | 1.0g |
![]()
x=12.0g
��Ʒ�н���þ�������ٷֺ���Ϊ�� ![]() =75.0%��
=75.0%��
�𣺣�1����Ʒ�н���þ�������ٷֺ���Ϊ75.0%����2���������������Ϊ36.5%��
�����㾫����������Ҫ�����˸��ݻ�ѧ��Ӧ����ʽ�ļ�������֪ʶ�㣬��Ҫ���ո����ʼ�������=ϵ������Է�������֮�Ȳ�����ȷ�����⣮

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�