��Ŀ����
ijͬѧ��������������Ϊ7%��������Һ������һƿ������Һ��Ϊ�ⶨƿ����Һ�����ʵ�����������ȡ����Һ73g���ձ��У�����12.5gʯ��ʯ��ǡ����ȫ��Ӧ���Ƶ��ձ���ʣ�����ʵ���������81.1g�������Ҫ��ش��������⣺��1��������ѧ��Ӧ�Ļ�ѧ����ʽΪ
��2�����������������
��3����������������г�����Ȼ��������ı���ʽΪ
��4��ƿ��������Һ�����ʵ�����������
��5����ȡһ������ƿ����Һ����������������Ϊ7%��������Һ50g�������ˮ��������g��
���𰸡�����������һ���ۺ��⣬������ѧ���������غ㶨�ɵ����⡢��ѧ����ʽ�ļ������д����ѧ����ʽ�ļ����Լ��������������ļ��㣬��ѵ����ѧ����Һϡ�͵ļ���������
����⣺��1��ϡ�����̼��Ʒ�Ӧ�����Ȼ��ơ�ˮ��������̼����ѧ����ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2�������Դ�Ϊ��CaCO3+2HCl=CaCl2+H2O+CO2��
��2������������������ڷ�Ӧǰ���ʵ������ܺͼ�ȥ��Ӧ���ձ������ʵ������ܺͣ�73g+12.5g-81.1g=4.4g���ʴ�Ϊ��4.4g
��3�����Ȼ��������Ϊx��
CaCO3+2HCl=CaCl2+H2O+CO2��
73 44
x 4.4g
�б���ʽ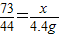
�ʴ�Ϊ
��4����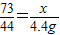 ���x=7.3g���������������������
���x=7.3g�������������������Ϊ��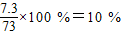 ���ʴ�Ϊ��10%
���ʴ�Ϊ��10%
��5��Ҫ���ˮ��������������Ҫԭ��Һ����������ԭ��Һ����Ϊx ��
50g×7%=x?10%
��� x=35g
���ˮ������Ϊ50g-35g=15g��
�ʴ�Ϊ��15g
�������ڣ�1����-�ڣ�4�����ǻ�����ѧ���ܹ�ͨ����˼���ȽϿ�Ĵ�������ڣ�5������һ������һ��˼ά��ȵ���Ŀ��Ҫ��˼����ˮ����ԭ��Һ�Ļ����ϼ�ˮ��50�ˣ���������Ҫ�����ԭ����Һ��������Ȼ���ӭ�ж����ˣ�
����⣺��1��ϡ�����̼��Ʒ�Ӧ�����Ȼ��ơ�ˮ��������̼����ѧ����ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2�������Դ�Ϊ��CaCO3+2HCl=CaCl2+H2O+CO2��
��2������������������ڷ�Ӧǰ���ʵ������ܺͼ�ȥ��Ӧ���ձ������ʵ������ܺͣ�73g+12.5g-81.1g=4.4g���ʴ�Ϊ��4.4g
��3�����Ȼ��������Ϊx��
CaCO3+2HCl=CaCl2+H2O+CO2��
73 44
x 4.4g
�б���ʽ
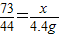
�ʴ�Ϊ

��4����
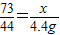 ���x=7.3g���������������������
���x=7.3g�������������������Ϊ��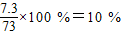 ���ʴ�Ϊ��10%
���ʴ�Ϊ��10%��5��Ҫ���ˮ��������������Ҫԭ��Һ����������ԭ��Һ����Ϊx ��
50g×7%=x?10%
��� x=35g
���ˮ������Ϊ50g-35g=15g��
�ʴ�Ϊ��15g
�������ڣ�1����-�ڣ�4�����ǻ�����ѧ���ܹ�ͨ����˼���ȽϿ�Ĵ�������ڣ�5������һ������һ��˼ά��ȵ���Ŀ��Ҫ��˼����ˮ����ԭ��Һ�Ļ����ϼ�ˮ��50�ˣ���������Ҫ�����ԭ����Һ��������Ȼ���ӭ�ж����ˣ�

��ϰ��ϵ�д�
 �ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д�
�ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д�
�����Ŀ