��Ŀ����
ˮ�DZ������Ȼ��Դ����һ����������������ģ�����Ӧ���˽��й�ˮ��һЩ֪ʶ����ش��������⣺
��1������ˮ������ˮ����Ȫˮ����ˮ�����ڴ��������______��
��2���þ�ˮϴ�·�ʱ���������������Ҳ�����������ԭ���Ǿ�ˮ������______��ѡ�Ӳˮ������ˮ������
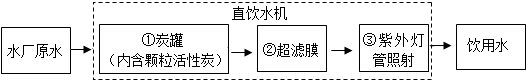
��3����ͼ��һ�ּ��û���������Һ�����������з�������Ҫ��Ӧ���Ȼ��ƺ�ˮ��ͨ�������·�Ӧ�����������ơ�������������Cl2�����÷�Ӧ�Ļ�ѧ����ʽΪ______���������仯�Ƕȿ����÷�Ӧ��______��ת��Ϊ��ѧ�ܣ�
��4������1000g������������15%��ʳ��ˮ����Ҫʳ��______g��Ҳ���������ʷ���25%��ʳ��ˮ______g��ˮϡ�Ͷ��ɣ�
��1������ˮ������ˮ����Ȫˮ����ˮ�����ڴ��������______��
��2���þ�ˮϴ�·�ʱ���������������Ҳ�����������ԭ���Ǿ�ˮ������______��ѡ�Ӳˮ������ˮ������
��3����ͼ��һ�ּ��û���������Һ�����������з�������Ҫ��Ӧ���Ȼ��ƺ�ˮ��ͨ�������·�Ӧ�����������ơ�������������Cl2�����÷�Ӧ�Ļ�ѧ����ʽΪ______���������仯�Ƕȿ����÷�Ӧ��______��ת��Ϊ��ѧ�ܣ�
��4������1000g������������15%��ʳ��ˮ����Ҫʳ��______g��Ҳ���������ʷ���25%��ʳ��ˮ______g��ˮϡ�Ͷ��ɣ�

��1������ˮ����Ȫˮ����ˮ�ж��ܽ����������ʣ����ڻ�������ˮ���ڴ����
��2��Ӳˮ����ˮ���������������ĸ�þ���ӵĶ��٣������п��÷���ˮ������Ӳˮ����ˮ��������ĭ�϶������ˮ�����ٵ���Ӳˮ���ʸþ�ˮ��Ӳˮ��
��3�������Ȼ��ƺ�ˮ��ͨ�������������������ơ�������������֪�÷�Ӧ�ķ���ʽΪ��2NaCl+2H2O
2NaOH+H2��+Cl2�����÷�Ӧ�ǽ�����ת��Ϊ��ѧ�ܣ�
��4��ʳ�ε�����Ϊ��1000g��15%=150g����Ҫ25%��ʳ��ˮ������Ϊ��
=600g��
�ʴ�Ϊ���磮��1������ˮ��2��Ӳˮ��3��2NaCl+2H2O
2NaOH+H2��+Cl2�����ܣ�4��150600
��2��Ӳˮ����ˮ���������������ĸ�þ���ӵĶ��٣������п��÷���ˮ������Ӳˮ����ˮ��������ĭ�϶������ˮ�����ٵ���Ӳˮ���ʸþ�ˮ��Ӳˮ��
��3�������Ȼ��ƺ�ˮ��ͨ�������������������ơ�������������֪�÷�Ӧ�ķ���ʽΪ��2NaCl+2H2O
| ||
��4��ʳ�ε�����Ϊ��1000g��15%=150g����Ҫ25%��ʳ��ˮ������Ϊ��
| 150g |
| 25% |
�ʴ�Ϊ���磮��1������ˮ��2��Ӳˮ��3��2NaCl+2H2O
| ||

��ϰ��ϵ�д�
�����Ŀ