��Ŀ����
ij������ȤС���һ������Ʒ���������ʣ����ʲ�����ˮ��Ҳ����ϡ���ᷴӦ�����з������ס��ҡ�����λͬѧ���ֱ����ʵ�飬����ֻ��һλͬѧ��ȡ�õ�ϡ����������Ʒǡ����ȫ��Ӧ��ʵ�����������| �� | �� | �� | |
| �ձ�+ϡ���� | 200g | 150g | 150g |
| ���������Ʒ | 9g | 9g | 14g |
| ��ַ�Ӧ�� �ձ�+ʣ���� | 208.7g | 158.7g | 163.7g |
��1����λͬѧ��ȡ�õ�ϡ����������Ʒǡ����ȫ��Ӧ��______��
��2���������Ʒ�����������������ðٷ�����ʾ��______��
���𰸡���������1���ɱ������ݷ�����֪��ͬѧ����ͬѧ��ʵ���������������ͬ������Ʒ������ͬ������ͬѧ�õ�ϡ���������࣬˵����ͬѧ��ʵ��ϡ�����������ͬѧ�ͱ�ͬѧ��ʵ����ϡ����������ͬ������Ʒ������ͬѧ�Ķ࣬������������������ͬ��˵����ͬѧ��ʵ������Ʒ���������ݴ˼����ж�˭����ǡ����ȫ��Ӧ��
��2����������Ѿ�֪������ͬѧ��ʵ����ǡ����ȫ��Ӧ�ģ����Լ���ʱҪ����ͬѧʵ������ݣ���������ϡ���ᷴӦ�Ļ�ѧ����ʽ���������������õ�����������
����⣺��1�������ϼ�ͬѧ����ͬѧ��ʵ�����ݷ�����֪��9g����Ʒ�ܲ���0.3g��������ͬѧ�ͱ�ͬѧ��ʵ�����ݷ�����֪��150gϡ�����ܲ���0.3g����������9g����Ʒ��150gϡ������ǡ����ȫ��Ӧ������ͬѧ��ʵ��ǡ����ȫ��Ӧ��
��2����������������150g+9 g-158.7 g=0.3 g
����Ʒ����������Ϊx
Fe+H2SO4�TFeSO4+H2��
56 2
x 0.3 g
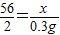
x=8.4 g
��Ʒ��������������Ϊ��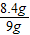 ×100%=93.3%
×100%=93.3%
�ʴ�Ϊ����1����ͬѧ����2��93%
�����������Ǹ��ݻ�ѧ����ʽ�ļ����⣬�������е��������Ա������ʽ�����ģ����Դ���Ĺؼ���ͨ���۲졢�����ͱȽϱ����еĶ������ݣ��ҳ��������������н��⣮
��2����������Ѿ�֪������ͬѧ��ʵ����ǡ����ȫ��Ӧ�ģ����Լ���ʱҪ����ͬѧʵ������ݣ���������ϡ���ᷴӦ�Ļ�ѧ����ʽ���������������õ�����������
����⣺��1�������ϼ�ͬѧ����ͬѧ��ʵ�����ݷ�����֪��9g����Ʒ�ܲ���0.3g��������ͬѧ�ͱ�ͬѧ��ʵ�����ݷ�����֪��150gϡ�����ܲ���0.3g����������9g����Ʒ��150gϡ������ǡ����ȫ��Ӧ������ͬѧ��ʵ��ǡ����ȫ��Ӧ��
��2����������������150g+9 g-158.7 g=0.3 g
����Ʒ����������Ϊx
Fe+H2SO4�TFeSO4+H2��
56 2
x 0.3 g
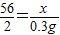
x=8.4 g
��Ʒ��������������Ϊ��
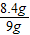 ×100%=93.3%
×100%=93.3%�ʴ�Ϊ����1����ͬѧ����2��93%
�����������Ǹ��ݻ�ѧ����ʽ�ļ����⣬�������е��������Ա������ʽ�����ģ����Դ���Ĺؼ���ͨ���۲졢�����ͱȽϱ����еĶ������ݣ��ҳ��������������н��⣮

��ϰ��ϵ�д�
�����Ŀ
ij������ȤС���һ������Ʒ���������ʣ����ʲ�����ˮ��Ҳ����ϡ���ᷴӦ�����з������ס��ҡ�����λͬѧ�ֱ����ʵ�飬����ֻ��һλͬѧ��ȡ�õ�ϡ����������Ʒǡ����ȫ��Ӧ��ʵ���������±���
��������������ݣ��ش��������⣺
��1�� ͬѧ��ȡ�õ�ϡ����������Ʒǡ����ȫ��Ӧ��
��2��������Ʒ����������������
��3������ǡ����ȫ��Ӧ��������Һ�����ʵ���������������������ȷ��0.1%��
| �� | �� | �� | |
| ϡ�����������g�� | 200 | 300 | 200 |
| ���������Ʒ��������g�� | 12 | 12 | 20 |
| ��ַ�Ӧ�����������������g�� | 0.4 | 0.4 | 0.4 |
��1��
��2��������Ʒ����������������
��3������ǡ����ȫ��Ӧ��������Һ�����ʵ���������������������ȷ��0.1%��
ij������ȤС���һ������Ʒ���������ʣ����ʲ�����ˮ��Ҳ����ϡ���ᷴӦ�����з������ס��ҡ�����λͬѧ�ֱ����ʵ�飬����ֻ��һλͬѧ��ȡ�õ�ϡ����������Ʒǡ����ȫ��Ӧ��ʵ���������£�
��������������ݣ��ش��������⣺���ձ�������Ϊ75.4g����������ȷ��1%��
��1�� ͬѧ��ȡ�õ�ϡ����������Ʒǡ����ȫ��Ӧ��
��2��������Ʒ����������������
��3������ϡ�������������������
| �� | �� | �� | |
| �ձ�+ϡ���� | 250g | 200g | 200g |
| ���������Ʒ | 9g | 9g | 14g |
| ��ַ�Ӧ���ձ�+ʣ���� | 258.7g | 208.7g | 213.7g |
��1��
��2��������Ʒ����������������
��3������ϡ�������������������