��Ŀ����
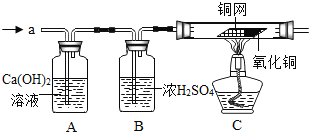
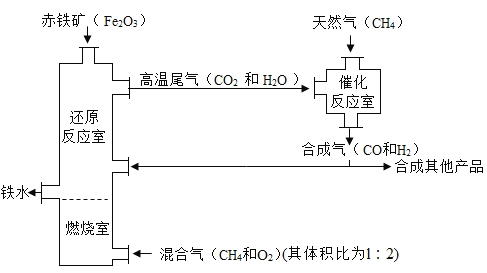
����Ŀ����¯ұ������������ͼ��ʾ��ʹ��Ȼ�������������������������ȼ������ʹ��������Ȼ��ȼ�ա��ڴ�����![]() ���Էֱ���
���Էֱ���![]() ��
��![]() ���þ�����
���þ�����![]() ��
��![]() ���Իش��������⣺
���Իش��������⣺
��1����ʯȼ�ϳ���Ȼ�������_________��_________�����й�����β������������CNG����־��д����CNG��������ȫ�ƣ�___________��
��2������Ʒ������ʴ������Ʒ����ʴ��ԭ��____________��
��3��ȼ���ҵ������ǣ�__________________��
��4���밴Ҫ��д������ʽ��
�ٻ�ԭ��Ӧ������ˮ�Ļ�ѧ��Ӧ����ʽ��________________________��
�ڴ���Ӧ����![]() ��
��![]() �ڸ��������µĻ�ѧ��Ӧ����ʽ��__________________��
�ڸ��������µĻ�ѧ��Ӧ����ʽ��__________________��
��5������500t��������80%�ij�����ʯ�������Ͽ���ұ����������4%������������Ϊ______t��������һλС����
���𰸡�ú ʯ�� ѹ����Ȼ�� ���������Ҫ������������е�������ˮֱ�ӽӴ������������ɶ�����ʣ�����Ʒ�������⣻���ڿ���������������Ӧ�����������һ�����ܵ���������Ĥ���Ӷ���ֹ����һ�����������������Ʒ������ʴ ȼ�ղ������� 3H2+Fe2O3![]() 2Fe+3H2O CH4+H2O
2Fe+3H2O CH4+H2O![]() CO+3H2 291.7t
CO+3H2 291.7t
��������
��1����ʯȼ�ϳ���Ȼ�������ú��ʯ�ͣ����й�����β������������CNG����־��д����CNG��������ȫ�ƣ�ѹ����Ȼ����
��2�����������Ҫ������������е�������ˮֱ�ӽӴ������������ɶ�����ʣ�����Ʒ�������⣻���ڿ���������������Ӧ�����������һ�����ܵ���������Ĥ���Ӷ���ֹ����һ�����������������Ʒ������ʴ��
��3������ȼ�շų��˴������ȣ��ܹ��������ṩ���� ����ȼ���ҡ��������ǣ�ȼ�ղ���������
��4���ٻ�ԭ��Ӧ������ˮ�ķ�Ӧ���������������ڸ��������·�Ӧ��������ˮ����Ӧ�Ļ�ѧ��Ӧ����ʽΪ��3H2+Fe2O3![]() 2Fe+3H2O��
2Fe+3H2O��
�ڴ���Ӧ����![]() ��
��![]() �ڸ�������������CO��H2����Ӧ�Ļ�ѧ��Ӧ����ʽΪ��CH4+H2O
�ڸ�������������CO��H2����Ӧ�Ļ�ѧ��Ӧ����ʽΪ��CH4+H2O![]() CO+3H2��
CO+3H2��
��5���裺�����Ͽ�������������4%������������Ϊx��
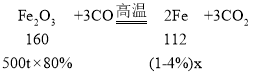
![]() x��291.7t��
x��291.7t��
�������Ͽ�������������4%��������������291.7t��

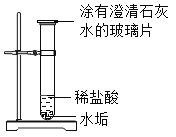
����Ŀ��I��ij����ˮ�к��н϶��̼�����[Ca(HCO3)2]������Ӳˮ�����ʱ����ˮ�����ɡ�ʵ��С����̽����ˮ������Ҫ�ɷ֣�
�����������������Ca(HCO3)2��ˮ��Ԫ����ɣ���ˮ������Ҫ�ɷֿ����ǣ�
��CaO ��Ca(OH)2 ��CaCO3 ��Ca(OH)2��CaCO3
Сǿͬѧ��Ϊ��ˮ���в������CaO��������___
��ʵ������ʵ��
ʵ��һ | ʵ������ | ���ͻ���� | ||
��������ˮ����ˮװ���Թ��У�������ã��õι�ȡ�ϲ���Һ��С�Թ��У�Ȼ������С�Թ��е�����ɫʯ����Һ | ��ɫʯ����Һ����ɫ | ����ɫ˵��ˮ����һ��û��___ | ||
ʵ��� | ʵ������ | ���ͻ���� | ||
| ��Ӧһ��ʱ���ˮ����ʧ����Һ���壻����Ƭ�ϳ���ʯ��ˮ����� | ʯ��ˮ����ǣ�˵����CO2���ɡ���ˮ����һ������_____ | ||
��ԭ������֣����û�ѧ����ʽ��ʾʵ������йط�Ӧ�Ļ�ѧ��Ӧԭ����
(1)____,(2)________��
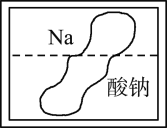
II����̽��������Ե�ʵ����ϣ�ͬѧ�ǰѴ�ĥ��������Ƭ��������ͭ��Һ�У���һ�����������һ��������������������Ƭ���濴���к�ɫ����������ͬʱ��������������������ð����д�����ɺ�ɫ����Ļ�ѧ����ʽ____��
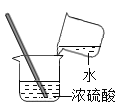
��������⣩��������ʲô�أ�
����������裩����һ��O2 �������H2 ��������CO2 �����ģ�SO2
ͬѧ�Ǿ������ۣ�һ����Ϊ��������������������___��
��ʵ����֤������ͬѧ����һ�¸����壬��������ζ��֤��������Ҳ��������������___��
�ƽ����������徭�鴿���õ��ܵ��뵽��ȼ�ľƾ��ƻ����ϣ����尲��ȼ�գ���������ɫ���森
��ʵ����ۣ�____��
����˼�뽻����CuSO4��Һ������____��(������������������������)��
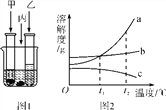
����Ŀ��������ۡ��ۺͷ���֮�����ϵ����Ҫ���塣
��1����ͼ�������ɲ�ͬ���ӹ��ɵģ����û�ѧ���ű�ʾ��������Щ���ʵ����ӡ�
|
|
|
���ɵ�������______ | ���ɵ�������______ | ���ɵ�������______ |
��2����ͼ��ʾΪijЩԪ�غ�ԭ�ӽṹ�IJ�����Ϣ��
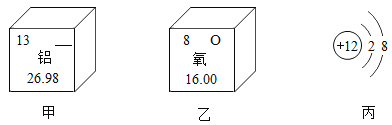
��ͼ��ʾԪ�ص�Ԫ�ط����� ______����ͼ��ʾԪ�ص�ԭ�ӵĺ˵������______�� ��ͼ��ʾ���������� ______���ԭ�ӡ����������ӡ��������ӡ�����
��3��N(NO2) 3�ǿ�ѧ�� 2011 �귢�ֵ�һ�����ͻ��ȼ�ϡ��Լ���N(NO2) 3�е�Ԫ�غ���Ԫ�ص�������Ϊ ______���������������ȱ�ʾ����N(NO2) 3�е�Ԫ�ص���������______�����ս������һλС������