��Ŀ����
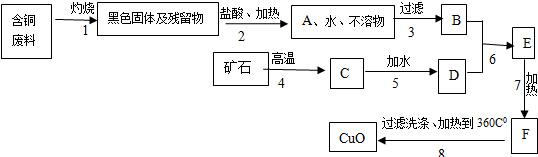
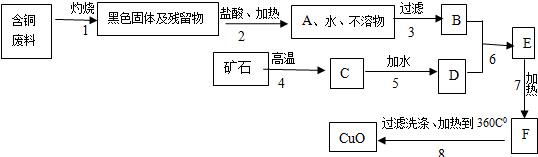
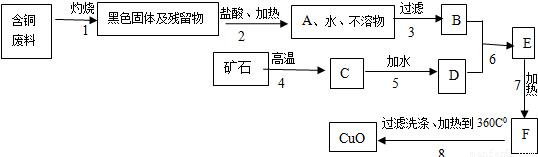
ij������������ͭ���ϣ�����������ͭ������������ͼ��
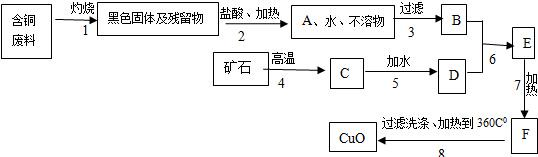
��֪��
�����������еĿ�ʯ���ֳ����Ľ������ϣ�
�����1�õ��IJ����ﲻ����ˮ��ϡ���
�����6��ǡ����ȫ��Ӧ��
��Cu��OH��2��ˮ���ǽ�״���������ѹ��ˣ�
��CaCl2���Ⱥ���ѷֽ⣬���ܽ�����¶����߶�����
��Cu��OH��2���Ⱥ��ֽ��CuO��H2O��
��1��A��C����Ҫ�ɷݵĻ�ѧʽ�ǣ�______��______��
��2������7�����ȵ���ҪĿ���ǣ�______��
��3��B��F���Ļ����ֱ�����______��______����д����Һ������Һ������Һ��
��4�������������1��7���Ļ�ѧ����ʽ��______��______
��5�����������Ҫ�Ƶ�ͭ����Ӧ��B�����������______��д��ѧʽ������Ҫ�����٣�д��B����������Ҫ��ѧ��Ӧ����ʽ��______��
�⣺��1��ͭ���պ�ĺ�ɫ����������ͭ������ͭ�����ᷴӦ�����Ȼ�ͭ������A�� CuCl2����ʯ�ǽ������ϣ��������գ���ô����̼��ƣ�̼������պ����� CaO��
��2������7�����ȵ���ҪĿ���� ������ͭ���ȷֽ���������ͭ����ΪCu��OH��2��ˮ���ǽ�״���������ѹ��ˣ����Ⱥ�������ͭ���ܹ����˷����ˣ�
��3��B�����Ȼ�ͭ��Һ��F�����в��ܵĹ��壬����������Һ��
��4����������1��7���Ļ�ѧ����ʽ��2Cu+O2 2CuO��Cu��OH��2
2CuO��Cu��OH��2 CuO+H2O
CuO+H2O
��5�����������Ҫ�Ƶ�ͭ����Ӧ��B����������� ����Ȼ����ˣ���Ϊ�����û����Ȼ�ͭ�е�ͭ����ѧ����ʽΪ Fe+CuCl2�TCu+FeCl2��
�𰸣���1��CuCl2��CaO��
��2��������ͭ���ȷֽ���������ͭ��
��3����Һ������Һ
��4��2Cu+O2 2CuO��Cu��OH��2
2CuO��Cu��OH��2 CuO+H2O
CuO+H2O
��5������Fe+CuCl2�TCu+FeCl2��
��������������ͭ��ȡ����ͭ����Ҫ�����ǣ��Ѻ����ϵ�ͭ���ճ�����ͭ��������ͭ�����������˵õ�ͭ������Һ��ͭ������Һ�����Һ��Ӧ��ȡ������ͭ������������ͭ�ֽ������ͭ��������ϴ�ӵõ�����������ͭ�������������֪�����ƶϣ���ʯ����Ҫ�ɷ���̼��ƣ����պ����������ƣ���������ˮ��Ӧ�����������ƣ�����������Һ���Ȼ�ͭ��Һ��Ӧ����������ͭ����������������ͭ��ֽ���������ͭ��
����������Ϻõ�ڹ�������ʵ���ȡ���̣����ӷ��������л�ѧ����ʽ����д�����⣬��������ͬѧ�ǵ����������ͷ���������
��2������7�����ȵ���ҪĿ���� ������ͭ���ȷֽ���������ͭ����ΪCu��OH��2��ˮ���ǽ�״���������ѹ��ˣ����Ⱥ�������ͭ���ܹ����˷����ˣ�
��3��B�����Ȼ�ͭ��Һ��F�����в��ܵĹ��壬����������Һ��
��4����������1��7���Ļ�ѧ����ʽ��2Cu+O2
 2CuO��Cu��OH��2
2CuO��Cu��OH��2 CuO+H2O
CuO+H2O��5�����������Ҫ�Ƶ�ͭ����Ӧ��B����������� ����Ȼ����ˣ���Ϊ�����û����Ȼ�ͭ�е�ͭ����ѧ����ʽΪ Fe+CuCl2�TCu+FeCl2��
�𰸣���1��CuCl2��CaO��
��2��������ͭ���ȷֽ���������ͭ��
��3����Һ������Һ
��4��2Cu+O2
 2CuO��Cu��OH��2
2CuO��Cu��OH��2 CuO+H2O
CuO+H2O��5������Fe+CuCl2�TCu+FeCl2��
��������������ͭ��ȡ����ͭ����Ҫ�����ǣ��Ѻ����ϵ�ͭ���ճ�����ͭ��������ͭ�����������˵õ�ͭ������Һ��ͭ������Һ�����Һ��Ӧ��ȡ������ͭ������������ͭ�ֽ������ͭ��������ϴ�ӵõ�����������ͭ�������������֪�����ƶϣ���ʯ����Ҫ�ɷ���̼��ƣ����պ����������ƣ���������ˮ��Ӧ�����������ƣ�����������Һ���Ȼ�ͭ��Һ��Ӧ����������ͭ����������������ͭ��ֽ���������ͭ��
����������Ϻõ�ڹ�������ʵ���ȡ���̣����ӷ��������л�ѧ����ʽ����д�����⣬��������ͬѧ�ǵ����������ͷ���������

��ϰ��ϵ�д�
�����Ŀ