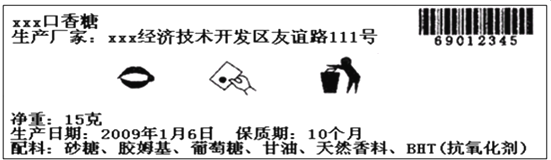
��Ŀ����
����Ŀ����֪���صĻ�ѧʽΪCO(NH2)2�������仯ѧʽ���㣺
(1)���ص���Է�������Ϊ___________��
(2)�����е����⡢̼����Ԫ�ص�������Ϊ___________
(3)�����е�Ԫ�ص�����������_____________
(4)12g�����к���Ԫ��___________g��
(5)12g�����еĺ�������___________g�����(NH4NO3)�еĺ�������ȡ�
(6)��һ����������Ʒ������ѧ������֪����37.4%(�����в�����Ԫ��)���Լ���� ������Ʒ��CO(NH2)2������������_________________��
���𰸡�60 7��1��3��4 46.7% 5.6g 16g 79.4%
��������
��1���������صĻ�ѧʽ��֪��������Է�������=12+16+14��2+1��4=60��
��2�������е����⡢̼����Ԫ�ص�������Ϊ��14��2������1��4����12��16=7��1��3��4��
��3�������е�Ԫ�ص���������=![]() ��100%��46.7%��
��100%��46.7%��
��4��12g�����к���Ԫ�ص�����Ϊ![]() g=5.6g��
g=5.6g��
��5�������(NH4NO3)�е�Ԫ�ص�����������![]() ��100%=35%������12���صĺ�����Ϊ5.6g����H����5.6g��Ԫ�ص�����淋�����Ϊ
��100%=35%������12���صĺ�����Ϊ5.6g����H����5.6g��Ԫ�ص�����淋�����Ϊ![]() =16g��
=16g��
��6����������Ʒ��CO(NH2)2������������![]() ��100%
��100%![]() 80.1%��
80.1%��