��Ŀ����
��2008?���ݣ������Զ��ͭ����������������ɫͭ�⣬����Ҫ�ɷ�Ϊ�������ʽ̼��ͭ[Cu2��OH��2CO3]���仯ѧ���������ڼ��̼��ƣ���Ҫ��ȥͭ�������ϵ�ͭ�⣬����ϡ�����ϡ�����뷴Ӧ������Һ�������ȥ��
��1��д��ϡ����ͼ�ʽ̼��ͭ��Ӧ�Ļ�ѧ����ʽ��
��2����ʽ���㣺2000��������������Ϊ2.5%��������Һ�����ԺͶ��ٿ˼�ʽ̼��ͭ��Ӧ��������1λС�������ԭ��������H-1��C-12��Cu-64��O-16��S-32��
��1��д��ϡ����ͼ�ʽ̼��ͭ��Ӧ�Ļ�ѧ����ʽ��
Cu2��OH��2C03+2H2S04=2CuS04+CO2��+3H2O
Cu2��OH��2C03+2H2S04=2CuS04+CO2��+3H2O
����2����ʽ���㣺2000��������������Ϊ2.5%��������Һ�����ԺͶ��ٿ˼�ʽ̼��ͭ��Ӧ��������1λС�������ԭ��������H-1��C-12��Cu-64��O-16��S-32��
��������1�����ݼ�ʽ̼��ͭ�߱����̼���ε����ʷ������������ᷴӦ�IJ��
��2���������ϵķ���ʽ�ͷ�Ӧ������������������Ӧ�ļ�ʽ̼��ͭ��������
��2���������ϵķ���ʽ�ͷ�Ӧ������������������Ӧ�ļ�ʽ̼��ͭ��������
����⣺��1�����ڼ�ʽ̼��ͭ�߱����̼���ε����ʣ�����[Cu2��OH��2CO3]�����ᷴӦ�IJ���������ͭ��ˮ�Ͷ�����̼������ʽΪCu2��OH��2C03+2H2S04=2CuS04+CO2��+3H2O��
��2���裺���Ժ�x�˼�ʽ̼��ͭ��Ӧ
Cu2��OH��2C03+2H2S04=2CuS04+CO2��+3H2O
222 196
x 2000g��2.5%
=
x=56.6g
�ʴ�Ϊ����1��Cu2��OH��2C03+2H2S04=2CuS04+CO2��+3H2O����2���𣺿��Ժ�56.6�˼�ʽ̼��ͭ��Ӧ��
��2���裺���Ժ�x�˼�ʽ̼��ͭ��Ӧ
Cu2��OH��2C03+2H2S04=2CuS04+CO2��+3H2O
222 196
x 2000g��2.5%
| 222 |
| x |
| 196 |
| 2000g��2.5% |
x=56.6g
�ʴ�Ϊ����1��Cu2��OH��2C03+2H2S04=2CuS04+CO2��+3H2O����2���𣺿��Ժ�56.6�˼�ʽ̼��ͭ��Ӧ��
�����������Ƕ���Ϣ���úͻ�ѧ����ʽ���㿼���⣬����Ĺؼ���������Ϣ��д����صķ���ʽ��

��ϰ��ϵ�д�
 ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�
�����Ŀ
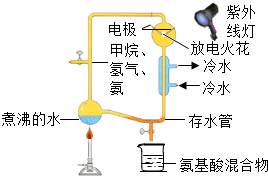 ��2008?���ݣ���ʷ�Ͼ����ǣ���ľ�Ǻ����ǣ��Ĵ����ɷֱ仯���ܱ�С�����������ǵĴ����ж�û��������������Ҫ�ɷ�������������������Ͱ����ɴ��ƶϣ�ԭʼ��������ɷֺ�ľ�ǡ������ϵĴ����ɷ����ƣ�1953����������һ��̽�������ϵ������������Դ��ʵ�飨��ͼ������ʵ���װ��ͼ����������װ����ͨ���Ʋ��ԭʼ�����ɷ֣����顢����������ˮ������ͨ���ŵ�����������ߵķ�������ԭʼ����Ļ�������������˶��ְ����ᣮ�������ͼ�ش��������⣺
��2008?���ݣ���ʷ�Ͼ����ǣ���ľ�Ǻ����ǣ��Ĵ����ɷֱ仯���ܱ�С�����������ǵĴ����ж�û��������������Ҫ�ɷ�������������������Ͱ����ɴ��ƶϣ�ԭʼ��������ɷֺ�ľ�ǡ������ϵĴ����ɷ����ƣ�1953����������һ��̽�������ϵ������������Դ��ʵ�飨��ͼ������ʵ���װ��ͼ����������װ����ͨ���Ʋ��ԭʼ�����ɷ֣����顢����������ˮ������ͨ���ŵ�����������ߵķ�������ԭʼ����Ļ�������������˶��ְ����ᣮ�������ͼ�ش��������⣺