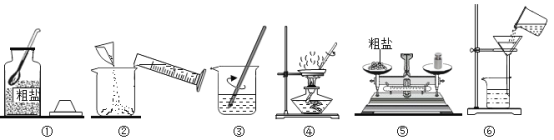
��Ŀ����
ijѧУ��֯ѧ��������Ұ����
��1����Щͬѧ�������µ�ʳƷ��������ҪΪ�����ṩ�����ʵ���_______________��
A ����
B ���
C ������
D ���
��2����ͬѧ���ı��ɰ����У�����ʯ��������������û�ѧ����ʽ��ʾ��ԭ��_______��
��3����Ұ������õ���ˮ����һ������ˮ�⣬����Ϊˮ���ˮ����___________����������������С��ͬѧ��Ϊ��ˮ���ˮ��Ӳˮ������Ϊ������_____________��������Ҫ�ⶨ��ˮ���ˮ�����ȣ�����ѡ��__________��
A ��ɫʯ����Һ
B ��ɫ��̪��Һ
C pH��ֽ
��4����Ұ��������У�С����С�ı��Ʒ����ˣ��Ʒ䶾Һ�ʼ��ԣ���������Ʒ��������ͿĨ�Լ�����ʹ����ͿĨ�Լ�����ʹ����_______������ĸ����
A ʳ��
B ʳ��ˮ
C ����ˮ
D ϴ�ྫ
��5��Ұ���õĹ��߰�����____________�ԣ�����ܡ����ȹ̡�����
��6�������г���֧�ܽ������ᴦ�����Է�ֹ�����⣬ԭ����_____________��
 ��ѧ�����ϵ�д�
��ѧ�����ϵ�д� �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д��г��ϳ�����һЩ�����ӷ���������ɫʳƷ������ʳƷ����һ��������װ�ı��ʼ�������Σ��ʳƷ��ȫ��ijͬѧ����һ���ڿ����з���һ��ʱ���ʳƷ���ʼ��������ijɷֽ���̽����
���������ϣ�
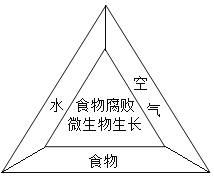
��1��ʳ�︯������Ϊ������ʳ������������ͼ��ʾ������ֻҪ�õ����֣��ټ��ϳ����������ˮ�����ʵ��������¾ͻ�Ѹ����������ˣ�ֻҪȱ��һ���������Ϳ��Է�ֹʳ�︯�ܡ�
��2�������ľ�����ˮ�Ե����������¼��֣���Ũ���� ����ʯ�� ���������ƹ��� �����ۢݹ轺����ѧʽ SiO2��nH2O����ɫ����״���壬������
��3�������Ȼ�����Һ�ڳ����·�����Ӧ�����Ȼ�������
�����̽����
��1�������жϣ�����Ϊ�١��ݵ������У����ʺ���ʳƷ����������ʼ�������________________��
��2��ʵ��̽����
ʵ�� | ��Ҫ���� | ��������� |
ʵ�� 1�������ֱ��ʼ��İ�װ����ȡ������Ʒ���Թ��У����й۲� | ����ƷΪ��ɫ���� | �ñ��ʼ�һ������____ |
ʵ�� 2����ʵ�� 1 ���Թ��м��������ϡ���ᣬ�� | �������ܽ⣬�����ݲ��� | �ñ��ʼ���һ�����е�������_____ |
ʵ�� 3����ȡ�ñ��ʼ���������ʵ��
| ����ʯ��ˮ����� |
�����˼��
��1��ʵ��2��ַ�Ӧ��������Һ�е�����һ����__________________��
��2�������ʳ�︯�ܵ��������������dz�����ʹ����Щ���ʼ��⣬�����Բ��õı��ʷ�����_____��д��һ�ּ��ɣ���
���ձ�����μ���X��Һ�����������ɳ�������������������X��Һ��������ϵ������ͼ����

ѡ�� | �ձ��е����� | X��Һ |
A | ϡHCl��Na2SO4 | BaCl2��Һ |
B | Cu��Fe | ϡHCl |
C | ϡH2SO4��MgCl2 | NaOH��Һ |
D | NaHCO3��K2SO4 | ϡHCl |
A.A B.B C.C D.D


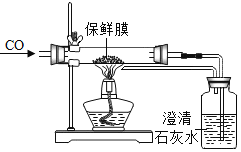
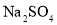 ��NaCl��
��NaCl��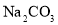 ��
��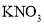 �е�һ�ֻ�����ɣ���ʵ�����£�
�е�һ�ֻ�����ɣ���ʵ�����£�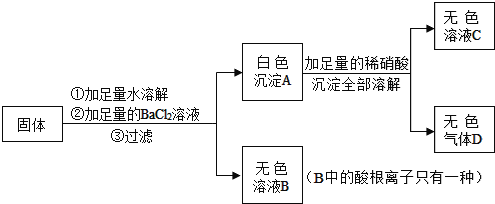
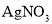 ��Һ���۲����ް�ɫ�������ɣ��ɴ˵ó����ۡ�������ijͬѧ��ʵ�鷽�����У�����������
��Һ���۲����ް�ɫ�������ɣ��ɴ˵ó����ۡ�������ijͬѧ��ʵ�鷽�����У����������� ��Һ��Ŀ����_____��
��Һ��Ŀ����_____��